The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system
- PMID: 18024991
- PMCID: PMC2919342
- DOI: 10.1093/toxsci/kfm283
The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system
Abstract
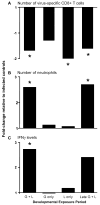
There is growing evidence that prenatal and early postnatal environmental factors influence the development and programming of the immune system, causing long-lasting negative health consequences. The aryl hydrocarbon receptor (AhR) is an important modulator of the development and function of the immune system; however, the mechanism is poorly understood. Exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin throughout gestation and during lactation yields adult offspring with persistent defects in their immune response to influenza virus. These functional alterations include suppressed lymphocyte responses and increased inflammation in the infected lung despite normal cellularity and anatomical development of lymphoid organs. The studies presented here were conducted to determine the critical period during immune ontogeny that is particularly sensitive to inappropriate AhR activation. We also investigated the contribution of AhR-mediated events within and extrinsic to hematopoietic cells. Our findings show that AhR activation alters different elements of the immune system at different times during development by affecting different tissue targets. In particular, diminished T-cell responses arise due to deregulated events within bone marrow-derived cells. In contrast, increased interferon gamma levels in the infected lung result from AhR-regulated events extrinsic to bone marrow-derived cells, and require AhR agonist exposure during early gestation. The persistence of AhR activation induced immune modulation was also compared, revealing that AhR activation causes long-lasting functional alterations in the developing immune system, whereas the impact on the mature immune system is transient.
Figures
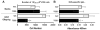

References
-
- Allan L, Mann K, Matulka R, Ryu H, Schlezinger J, Sherr D. Bone marrow stromal-B cell interactions in polycyclic aromatic hydrocarbon-induced pro/pre-B cell apoptosis. Toxicol Sci. 2003;76:357–365. - PubMed
-
- Andersson P, Ridderstad A, McGuire J, Pettersson S, Poellinger L, Hanberg A. A constitutively active aryl hydrocarbon receptor causes loss of peritoneal B1 cells. Biochem Biophys Res Commun. 2003;302:236–241. - PubMed
-
- Birnbaum L. Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab Dispos. 1986;14:34–40. - PubMed
-
- Camacho I, Nagarkatti M, Nagarkatti P. Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2004;78:96–106. - PubMed
-
- Camacho I, Singh N, Hegde V, Nagarkatti M, Nagarkatti P. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappa B and expression of fas ligand in thymic stromal cells and consequent T cell apoptosis. J Immunol. 2005;175:90–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

