SUMO1 negatively regulates BRCA1-mediated transcription, via modulation of promoter occupancy
- PMID: 18025037
- PMCID: PMC2248730
- DOI: 10.1093/nar/gkm969
SUMO1 negatively regulates BRCA1-mediated transcription, via modulation of promoter occupancy
Abstract
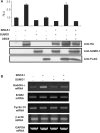
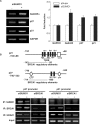
BRCA1, a tumor suppressor gene, is implicated in the repression and activation of transcription via interactions with a diverse range of proteins. The mechanisms regulating the action of BRCA1 are not fully understood. Here, we use the promoters of Gadd45alpha, p27(KIP1) and p21(WAF1/CIP1) to demonstrate that SUMO1 represses transactivation potential of BRCA1 by causing BRCA1 to be released from the promoters and augmenting histone deacetylation via recruitment of histone deacetylase (HDAC) activity. Consistently, silencing of SUMO1 led to recruitment of BRCA1 and release of HDAC1 at the BRCA1 target promoters, and subsequent transcriptional activation of the BRCA1 target genes. Furthermore, a sumoylation-incompetent mutant missing the sumoylation donor site suppressed BRCA1-induced activation of transcription, whereas E2 UBC9 or the dominant-negative mutant UBC9 had no effect, implying that repression of BRCA1-mediated activation of transcription by SUMO1 is independent of sumoylation. Repression of BRCA1-mediated activation of transcription by SUMO1 was reversed by DNA damage by inducing the release of SUMO1 from the Gadd45alpha promoter and the recruitment of BRCA1, along with increased histone acetylation, to enhance activation of transcription. Together, our data provide evidence that SUMO1 plays a role in the activation-repression switch of BRCA1-mediated transcription via modulation of promoter occupancy.
Figures











References
-
- Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001;10:705–713. - PubMed
-
- Venkitaraman AR. Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J Cell Sci. 2001;114:3591–3598. - PubMed
-
- Zheng L, Pan H, Li S, Flesken-Nikitin A, Chen PL, Boyer TG, Lee WH. Sequence specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol. Cell. 2000;6:757–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

