yadBC of Yersinia pestis, a new virulence determinant for bubonic plague
- PMID: 18025093
- PMCID: PMC2223446
- DOI: 10.1128/IAI.00219-07
yadBC of Yersinia pestis, a new virulence determinant for bubonic plague
Erratum in
-
Partial Retraction: yadBC of Yersinia pestis, a new virulence determinant for bubonic plague.Infect Immun. 2013 Feb;81(2):619. doi: 10.1128/IAI.01279-12. Infect Immun. 2013. PMID: 23335642 Free PMC article. No abstract available.
Abstract
In all Yersinia pestis strains examined, the adhesin/invasin yadA gene is a pseudogene, yet Y. pestis is invasive for epithelial cells. To identify potential surface proteins that are structurally and functionally similar to YadA, we searched the Y. pestis genome for open reading frames with homology to yadA and found three: the bicistronic operon yadBC (YPO1387 and YPO1388 of Y. pestis CO92; y2786 and y2785 of Y. pestis KIM5), which encodes two putative surface proteins, and YPO0902, which lacks a signal sequence and likely is nonfunctional. In this study we characterized yadBC regulation and tested the importance of this operon for Y. pestis adherence, invasion, and virulence. We found that loss of yadBC caused a modest loss of invasiveness for epithelioid cells and a large decrease in virulence for bubonic plague but not for pneumonic plague in mice.
Figures
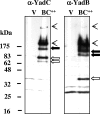
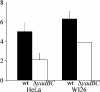
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2001. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
-
- Boyle, E. C., and B. B. Finlay. 2003. Bacterial pathogenesis: exploiting cellular adherence. Curr. Opin. Cell Biol. 15633-639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

