Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein
- PMID: 18025094
- PMCID: PMC2223467
- DOI: 10.1128/IAI.01125-07
Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein
Abstract
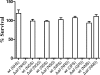
Yersinia pestis, the causative agent of plague, must survive in blood in order to cause disease and to be transmitted from host to host by fleas. Members of the Ail/Lom family of outer membrane proteins provide protection from complement-dependent killing for a number of pathogenic bacteria. The Y. pestis KIM genome is predicted to encode four Ail/Lom family proteins. Y. pestis mutants specifically deficient in expression of each of these proteins were constructed using lambda Red-mediated recombination. The Ail outer membrane protein was essential for Y. pestis to resist complement-mediated killing at 26 and 37 degrees C. Ail was expressed at high levels at both 26 and 37 degrees C, but not at 6 degrees C. Expression of Ail in Escherichia coli provided protection from the bactericidal activity of complement. High-level expression of the three other Y. pestis Ail/Lom family proteins (the y1682, y2034, and y2446 proteins) provided no protection against complement-mediated bacterial killing. A Y. pestis ail deletion mutant was rapidly killed by sera obtained from all mammals tested except mouse serum. The role of Ail in infection of mice, Caenorhabditis elegans, and fleas was investigated.
Figures
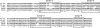


References
-
- Anisimov, A. P., S. V. Dentovskaya, G. M. Titareva, I. V. Bakhteeva, R. Z. Shaikhutdinova, S. V. Balakhonov, B. Lindner, N. A. Kocharova, S. N. Senchenkova, O. Holst, G. B. Pier, and Y. A. Knirel. 2005. Intraspecies and temperature-dependent variations in susceptibility of Yersinia pestis to the bactericidal action of serum and to polymyxin B. Infect. Immun. 737324-7331. - PMC - PubMed
-
- Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52451-469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

