Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia
- PMID: 18025095
- PMCID: PMC2223482
- DOI: 10.1128/IAI.00862-07
Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia
Abstract
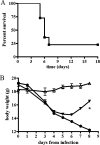
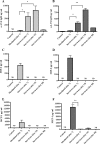
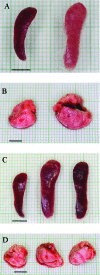
Francisella tularensis can cause severe disseminated disease after respiratory infection. The identification of factors involved in mortality or recovery following induction of tularemia in the mouse will improve our understanding of the natural history of this disease and facilitate future evaluation of vaccine candidate preparations. BALB/c mice were infected intranasally with the live vaccine strain (LVS) of F. tularensis subsp. holarctica and euthanized at different stages of disease to analyze the induction of immune molecules, gross anatomical features of organs, bacterial burdens, and progression of the histopathological changes in lung and spleen. Tissue-specific interleukin-6 (IL-6), macrophage inflammatory protein 2, and monocyte chemotactic protein 1 were immune markers of mortality, while anti-LVS immunoglobulin M and IL-1beta were associated with survival. Moribund mice had enlarged spleens and lungs, while surviving mice had even more prominent splenomegaly and normal-appearing lungs. Histopathology of the spleens of severely ill mice was characterized by disrupted lymphoid follicles and fragmented nuclei, while the spleens of survivors appeared healthy but with increased numbers of megakaryocytes and erythrocytes. Histopathology of the lungs of severely ill mice indicated severe pneumonia. Lungs of survivors at early time points showed increased inflammation, while at late times they appeared healthy with peribronchial lymphoid aggregates. Our results suggest that host immune factors are able to affect bacterial dissemination after respiratory tularemia, provide new insights regarding the pathological characteristics of pulmonary tularemia leading to systemic disease, and potentially identify immune markers associated with recovery from the disease.
Figures



References
-
- Bolger, C. E., C. A. Forestal, J. K. Italo, J. L. Benach, and M. B. Furie. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77893-897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

