Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection
- PMID: 18025098
- PMCID: PMC2223466
- DOI: 10.1128/IAI.01064-07
Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection
Abstract
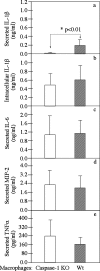
Chlamydia trachomatis infection induces inflammatory pathologies in the upper genital tract, potentially leading to ectopic pregnancy and infertility in the affected women. Caspase-1 is required for processing and release of the inflammatory cytokines interleukin-1beta (IL-1beta), IL-18, and possibly IL-33. In the present study, we evaluated the role of caspase-1 in chlamydial infection and pathogenesis. Although chlamydial infection induced caspase-1 activation and processing of IL-1beta, mice competent and mice deficient in caspase-1 experienced similar courses of chlamydial infection in their urogenital tracts, suggesting that Chlamydia-activated caspase-1 did not play a significant role in resolution of chlamydial infection. However, when genital tract tissue pathologies were examined, the caspase-1-deficient mice displayed much reduced inflammatory damage. The reduction in inflammation was most obvious in the fallopian tube tissue. These observations demonstrated that although caspase-1 is not required for controlling chlamydial infection, caspase-1-mediated responses can exacerbate the Chlamydia-induced inflammatory pathologies in the upper genital tract, suggesting that the host caspase-1 may be targeted for selectively attenuating chlamydial pathogenicity without affecting the host defense against chlamydial infection.
Figures

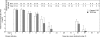

References
-
- Bauwens, J. E., H. Orlander, M. P. Gomez, M. Lampe, S. Morse, W. E. Stamm, R. Cone, R. Ashley, P. Swenson, and K. K. Holmes. 2002. Epidemic Lymphogranuloma venereum during epidemics of crack cocaine use and HIV infection in the Bahamas. Sex. Transm. Dis. 29253-259. - PubMed
-
- Bilenki, L., S. Wang, J. Yang, Y. Fan, A. G. Joyee, and X. Yang. 2005. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J. Immunol. 1753197-3206. - PubMed
-
- Boraschi, D., and A. Tagliabue. 2006. The interleukin-1 receptor family. Vitam. Horm. 74229-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

