Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection
- PMID: 18025112
- PMCID: PMC2224775
- DOI: 10.1128/AAC.00761-07
Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection
Abstract
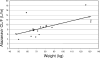
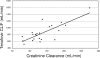
The primary objective of this study was to measure atazanavir-ritonavir and tenofovir pharmacokinetics when the drugs were used in combination in young adults with human immunodeficiency virus (HIV). HIV-infected subjects > or =18 to <25 years old receiving (> or =28 days) 300/100 mg atazanavir-ritonavir plus 300 mg tenofovir disoproxil fumarate (TDF) plus one or more other nucleoside analogs underwent intensive 24-h pharmacokinetic studies following a light meal. Peripheral blood mononuclear cells were obtained at 1, 4, and 24 h postdose for quantification of intracellular tenofovir diphosphate (TFV-DP) concentrations. Twenty-two subjects were eligible for analyses. The geometric mean (95% confidence interval [CI]) atazanavir area under the concentration-time curve from 0 to 24 h (AUC(0-24)), maximum concentration of drug in serum (C(max)), concentration at 24 h postdose (C(24)), and total apparent oral clearance (CL/F) values were 35,971 ng x hr/ml (30,853 to 41,898), 3,504 ng/ml (2,978 to 4,105), 578 ng/ml (474 to 704), and 8.3 liter/hr (7.2 to 9.7), respectively. The geometric mean (95% CI) tenofovir AUC(0-24), C(max), C(24), and CL/F values were 2,762 ng.hr/ml (2,392 to 3,041), 254 ng/ml (221 to 292), 60 ng/ml (52 to 68), and 49.2 liter/hr (43.8 to 55.3), respectively. Body weight was significantly predictive of CL/F for all three drugs. For every 10-kg increase in weight, there was a 10%, 14.8%, and 6.8% increase in the atazanavir, ritonavir, and tenofovir CL/F, respectively (P < or = 0.01). Renal function was predictive of tenofovir CL/F. For every 10 ml/min increase in creatinine clearance, there was a 4.6% increase in tenofovir CL/F (P < 0.0001). The geometric mean (95% CI) TFV-DP concentrations at 1, 4, and 24 h postdose were 96.4 (71.5 to 130), 93.3 (68 to 130), and 92.7 (70 to 123) fmol/million cells. There was an association between renal function, tenofovir AUC, and tenofovir C(max) and intracellular TFV-DP concentrations, although none of these associations reached statistical significance. In these HIV-infected young adults treated with atazanavir-ritonavir plus TDF, the atazanavir AUC was similar to those of older adults treated with the combination. Based on data for healthy volunteers, a higher tenofovir AUC may have been expected, but was not seen in these subjects. This might be due to faster tenofovir CL/F because of higher creatinine clearance in this age group. Additional studies of the exposure-response relationships of this regimen in children, adolescents, and adults would advance our knowledge of its pharmacodynamic properties.
Figures

References
-
- Agarwala, S., T. Eley, C. Villegas, Y. Wang, E. Hughes, J. Xie, and D. Grasela. 2005. Pharmacokinetic interaction between tenofovir and atazanavir coadministered with ritonavir in healthy subjects, abstr. 16. Sixth Int. Workshop Clin. Pharmacol. HIV Ther., Quebec City, Quebec, Canada, 26 to 29 April 2005.
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
-
- Bertz, R., Y. Wang, L. Mahnke, A. Persson, E. Chung, M. Mathew, S. Agarwala, D. Filoramo, J. Hammond, and D. Grasela. 2007. Assessment of pharmacokinetic/pharmacodynamic relationships through 48 weeks from a study in HIV+, antiretroviral-naive subjects receiving antiretroviral regimens containing atazanavir 400 mg or atazanavir/ritonavir 300/100 mg once daily, Abstr. 565. Fourteenth Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007.
-
- Chiba, K., T. Ishizaki, H. Miura, and K. Minagawa. 1980. Michaelis-Menten pharmacokinetics of diphenylhydantoin and application in the pediatric age patient. J. Pediatr. 96:479-484. - PubMed
-
- Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

