NKp30 ligation induces rapid activation of the canonical NF-kappaB pathway in NK cells
- PMID: 18025182
- PMCID: PMC4349331
- DOI: 10.4049/jimmunol.179.11.7385
NKp30 ligation induces rapid activation of the canonical NF-kappaB pathway in NK cells
Abstract
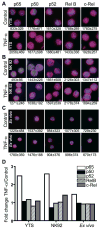
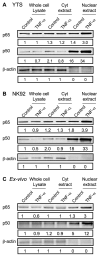
Studies of patients with congenital immunodeficiency due to mutation of the NF-kappaB essential modulator (NEMO) gene have demonstrated that NEMO integrity is required for NK cell cytotoxicity. Thus, we have studied the physiology of NF-kappaB activation in NK cells during the cytolytic program. In resting ex vivo human NK cells or cell lines, IkappaB was degraded after 10 min exposure to PMA and ionomycin, or TNF and was maximally degraded by 30 min. Ligation of several NK cell activation receptors including NKp30 induced a similar response and was blocked by pretreatment with the proteosome inhibitor MG132. There was no short-term effect on p100 processing, the signature of noncanonical NF-kappaB activation. NK cell IkappaB degradation corresponded to increases in nuclear NF-kappaB as detected by EMSA. Supershift of stimulated NK cells and fluorescence microscopy of individual NK cells in cytolytic conjugates demonstrated that the p65/p50 heterodimer was the primary NF-kappaB used. NF-kappaB function was evaluated in NK92 cells transduced with a kappaB GFP reporter, and their conjugation with K562 cells or ligation of NKp30 ligation resulted in rapid GFP accumulation. The latter was prevented by the Syk inhibitor piceatannol. Thus, NK cell activation signaling specifically induces transcriptional activation and synthesis of new NF-kappaB dependent proteins during the initiation of cytotoxicity.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



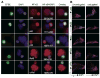
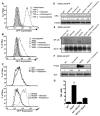
References
-
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. - PubMed
-
- Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–1516. - PMC - PubMed
-
- Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–6780. - PubMed
-
- Scheidereit C. IkappaB kinase complexes: gateways to NF-κB activation and transcription. Oncogene. 2006;25:6685–6705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

