Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum
- PMID: 18025266
- PMCID: PMC2134770
- DOI: 10.1101/gr.6714008
Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum
Abstract
Neuropeptides and protein hormones are ancient molecules that mediate cell-to-cell communication. The whole genome sequence from the red flour beetle Tribolium castaneum, along with those from other insect species, provides an opportunity to study the evolution of the genes encoding neuropeptide and protein hormones. We identified 41 of these genes in the Tribolium genome by using a combination of bioinformatic and peptidomic approaches. These genes encode >80 mature neuropeptides and protein hormones, 49 peptides of which were experimentally identified by peptidomics of the central nervous system and other neuroendocrine organs. Twenty-three genes have orthologs in Drosophila melanogaster: Sixteen genes in five different groups are likely the result of recent gene expansions during beetle evolution. These five groups contain peptides related to antidiuretic factor-b (ADF-b), CRF-like diuretic hormone (DH37 and DH47 of Tribolium), adipokinetic hormone (AKH), eclosion hormone, and insulin-like peptide. In addition, we found a gene encoding an arginine-vasopressin-like (AVPL) peptide and one for its receptor. Both genes occur only in Tribolium and not in other holometabolous insects with a sequenced genome. The presence of many additional osmoregulatory peptides in Tribolium agrees well with its ability to live in very dry surroundings. In contrast to these extra genes, there are at least nine neuropeptide genes missing in Tribolium, including the genes encoding the prepropeptides for corazonin, kinin, and allatostatin-A. The cognate receptor genes for these three peptides also appear to be absent in the Tribolium genome. Our analysis of Tribolium indicates that, during insect evolution, genes for neuropeptides and protein hormones are often duplicated or lost.
Figures


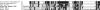



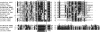
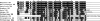
References
-
- Baernholdt D., Anderson S.O., Anderson S.O. Sequence studies on post-ecdysial cuticular proteins from pupae of the yellow mealworm, Tenebrio molitor. Insect Biochem. Mol. Biol. 1998;28:517–526. - PubMed
-
- Baggerman G., Cerstiaens A., De Loof A., Schoofs L., Cerstiaens A., De Loof A., Schoofs L., De Loof A., Schoofs L., Schoofs L. Peptidomics of the larval Drosophila melanogaster central nervous system. J. Biol. Chem. 2002;277:40368–40374. - PubMed
-
- Bendtsen J.D., Nielsen H., von Heijne G., Brunak S., Nielsen H., von Heijne G., Brunak S., von Heijne G., Brunak S., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. - PubMed
-
- Brown M.R., Graf R., Swiderek K.M., Fendley D., Stracker T.H., Champagne D.E., Lea A.O., Graf R., Swiderek K.M., Fendley D., Stracker T.H., Champagne D.E., Lea A.O., Swiderek K.M., Fendley D., Stracker T.H., Champagne D.E., Lea A.O., Fendley D., Stracker T.H., Champagne D.E., Lea A.O., Stracker T.H., Champagne D.E., Lea A.O., Champagne D.E., Lea A.O., Lea A.O. Identification of a steroidogenic neurohormone in female mosquitoes. J. Biol. Chem. 1998;273:3967–3971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
