A role for DNA-mediated charge transport in regulating p53: Oxidation of the DNA-bound protein from a distance
- PMID: 18025460
- PMCID: PMC2141881
- DOI: 10.1073/pnas.0709326104
A role for DNA-mediated charge transport in regulating p53: Oxidation of the DNA-bound protein from a distance
Abstract
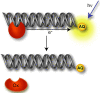
Charge transport (CT) through the DNA base pairs provides a means to promote redox reactions at a remote site and potentially to effect signaling between molecules bound to DNA. Here we describe the oxidation of a cell-cycle regulatory protein, p53, from a distance through DNA-mediated CT. A consensus p53 binding site as well as three DNA promoters regulated by p53 were synthesized containing a tethered DNA photooxidant, anthraquinone. Photoinduced oxidation of the protein occurs from a distance; introduction of an intervening CA mismatch, which inhibits DNA-mediated CT, prevents oxidation of p53. DNA-mediated oxidation is shown to promote dissociation of p53 from only some promoters, and this sequence-selectivity in oxidative dissociation correlates with the biological regulation of p53. Under severe oxidative stress, effected here through oxidation at long range, p53 dissociates from a promoter that activates DNA repair as well as the promoter for the negative regulator of p53, Mdm2, but not from a promoter activating cell-cycle arrest. Mass spectrometry results are consistent with disulfide bond formation in p53 upon DNA-mediated oxidation. Furthermore, DNA-bound p53 oxidation is shown in vivo by up-regulation of p53 and subsequent irradiation in the presence of a rhodium photooxidant to give a new p53 adduct that can be reversed with thiol treatment. This DNA-mediated oxidation of p53 parallels that seen by treating cells with hydrogen peroxide. These results indicate a unique mechanism using DNA-mediated CT chemistry by which p53 activity on different promoters may be controlled globally under conditions of oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



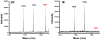


References
-
- Vogelstein B, Lane D, Levine AJ. Nature. 2000;408:307–310. - PubMed
-
- Vousden KH, Lu X. Nat Rev Cancer. 2002;2:594–604. - PubMed
-
- Prives C, Hall PA. J Pathol. 1999;187:112–126. - PubMed
-
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

