Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes
- PMID: 18025464
- PMCID: PMC2141895
- DOI: 10.1073/pnas.0707428104
Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes
Abstract
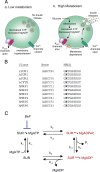
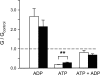
Gain-of-function mutations in the genes encoding the ATP-sensitive potassium (K(ATP)) channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) are a common cause of neonatal diabetes mellitus. Here we investigate the molecular mechanism by which two heterozygous mutations in the second nucleotide-binding domain (NBD2) of SUR1 (R1380L and R1380C) separately cause neonatal diabetes. SUR1 is a channel regulator that modulates the gating of the pore formed by Kir6.2. K(ATP) channel activity is inhibited by ATP binding to Kir6.2 but is stimulated by MgADP binding, or by MgATP binding and hydrolysis, at the NBDs of SUR1. Functional analysis of purified NBD2 showed that each mutation enhances MgATP hydrolysis by purified isolated fusion proteins of maltose-binding protein and NBD2. Inhibition of ATP hydrolysis by MgADP was unaffected by mutation of R1380, but inhibition by beryllium fluoride (which traps the ATPase cycle in the prehydrolytic state) was reduced. MgADP-dependent activation of K(ATP) channel activity was unaffected. These data suggest that the R1380L and R1380C mutations enhance the off-rate of P(i), thereby enhancing the hydrolytic rate. Molecular modeling studies supported this idea. Because mutant channels were inhibited less strongly by MgATP, this would increase K(ATP) currents in pancreatic beta cells, thus reducing insulin secretion and producing diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
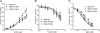

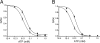

References
-
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. N Engl J Med. 2004;350:1838–1849. - PubMed
-
- Hattersley AT, Ashcroft FM. Diabetes. 2005;54:2503–2513. - PubMed
-
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Nature. 1997;387:179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

