Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism
- PMID: 18026094
- PMCID: PMC2727650
- DOI: 10.1038/nchembio.2007.54
Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism
Abstract
Bone morphogenetic protein (BMP) signals coordinate developmental patterning and have essential physiological roles in mature organisms. Here we describe the first known small-molecule inhibitor of BMP signaling-dorsomorphin, which we identified in a screen for compounds that perturb dorsoventral axis formation in zebrafish. We found that dorsomorphin selectively inhibits the BMP type I receptors ALK2, ALK3 and ALK6 and thus blocks BMP-mediated SMAD1/5/8 phosphorylation, target gene transcription and osteogenic differentiation. Using dorsomorphin, we examined the role of BMP signaling in iron homeostasis. In vitro, dorsomorphin inhibited BMP-, hemojuvelin- and interleukin 6-stimulated expression of the systemic iron regulator hepcidin, which suggests that BMP receptors regulate hepcidin induction by all of these stimuli. In vivo, systemic challenge with iron rapidly induced SMAD1/5/8 phosphorylation and hepcidin expression in the liver, whereas treatment with dorsomorphin blocked SMAD1/5/8 phosphorylation, normalized hepcidin expression and increased serum iron levels. These findings suggest an essential physiological role for hepatic BMP signaling in iron-hepcidin homeostasis.
Figures

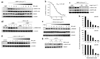

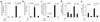
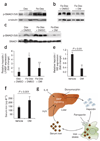
Comment in
-
Small-molecule dissection of BMP signaling.Nat Chem Biol. 2008 Jan;4(1):15-6. doi: 10.1038/nchembio0108-15. Nat Chem Biol. 2008. PMID: 18084273 No abstract available.
References
-
- Nguyen VH, et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. - PubMed
-
- Mullins MC, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. - PubMed
-
- Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev. Biol. 1999;214:181–196. - PubMed
-
- Mintzer KA, et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. - PubMed
-
- Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL079267/HL/NHLBI NIH HHS/United States
- R01 HL074352/HL/NHLBI NIH HHS/United States
- R01 CA118498/CA/NCI NIH HHS/United States
- CA118498/CA/NCI NIH HHS/United States
- K08 HL079943/HL/NHLBI NIH HHS/United States
- DK075846/DK/NIDDK NIH HHS/United States
- K08 HL081535/HL/NHLBI NIH HHS/United States
- HL081535/HL/NHLBI NIH HHS/United States
- HL079943/HL/NHLBI NIH HHS/United States
- R01 DK071837/DK/NIDDK NIH HHS/United States
- K08 DK075846/DK/NIDDK NIH HHS/United States
- DK071837/DK/NIDDK NIH HHS/United States
- R01 HL079267/HL/NHLBI NIH HHS/United States
- HL074352/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

