Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards
- PMID: 18026098
- PMCID: PMC2562672
- DOI: 10.1038/nn2013
Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards
Abstract
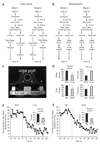
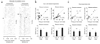
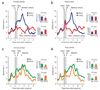
The dopamine system is thought to be involved in making decisions about reward. Here we recorded from the ventral tegmental area in rats learning to choose between differently delayed and sized rewards. As expected, the activity of many putative dopamine neurons reflected reward prediction errors, changing when the value of the reward increased or decreased unexpectedly. During learning, neural responses to reward in these neurons waned and responses to cues that predicted reward emerged. Notably, this cue-evoked activity varied with size and delay. Moreover, when rats were given a choice between two differently valued outcomes, the activity of the neurons initially reflected the more valuable option, even when it was not subsequently selected.
Figures



References
-
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. - PubMed
-
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. - PubMed
-
- Dayan P, Balleine BW. Reward, motivation and reinforcement learning. Neuron. 2002;36:285–298. - PubMed
-
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 2007;10:1020–1028. - PubMed
-
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J. Neurophysiol. 1994;72:1024–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

