Crohn's disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli
- PMID: 18028374
- PMCID: PMC2433311
- DOI: 10.1111/j.1365-2567.2007.02735.x
Crohn's disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli
Abstract
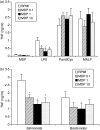
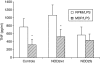
Mutations in nucleotide-binding oligomerization domain-2 (NOD2), leading to defective recognition of bacterial peptidoglycans, are associated with Crohn's disease. The underlying mechanism that results in increased inflammation in the guts of the patients bearing NOD2 mutations is still unclear. We hypothesized that NOD2 engagement leads to cross-tolerance to stimulation of Toll-like receptors (TLR), and we investigated whether patients with Crohn's disease who bear NOD2 mutations display a disturbed NOD2/TLR cross-tolerance. Peripheral blood mononuclear cells preincubated with NOD2 ligands were specifically down-regulated for the production of tumour necrosis factor-alpha (TNF-alpha) induced by the TLR4 ligand lipopolysaccharide, as well as by intestinal microorganisms, whereas the production of anti-inflammatory cytokines was not modulated. While in cells isolated from patients with Crohn's disease with the wild-type NOD2 allele, the NOD2 engagement led to a similar cross-tolerance to TLR4-dependent stimulation of TNF-alpha, the cross-tolerance between NOD2 and TLR4 was absent in the cells of five patients homozygous for the 3020insC NOD2 mutation, leading to uninhibited release of TNF-alpha by TLR4 ligands and intestinal bacteria. In conclusion, we propose the absence of NOD2/TLR4 cross-tolerance as a central mechanism for the increased susceptibility to Crohn's disease in individuals with NOD2 mutations.
Figures


References
-
- Hugot J-P, Laurent-Puig P, Gower-Rousseau C, et al. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature. 1996;379:821–3. - PubMed
-
- Hugot J-P, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. - PubMed
-
- Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD15) genotype with clinical course of Crohn’s disease: a cohort study. Lancet. 2002;359:1661–5. - PubMed
-
- Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. - PubMed
-
- Gutierrez O, Pipaon C, Inohara N, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-κB activation. J Biol Chem. 2002;277:41701–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

