Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study
- PMID: 18028431
- PMCID: PMC2268958
- DOI: 10.1111/j.1600-0609.2007.00985.x
Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study
Abstract
Objectives/methods: This 1-yr prospective phase II trial evaluated the efficacy of deferasirox in regularly transfused patients aged 3-81 yrs with myelodysplastic syndromes (MDS; n = 47), Diamond-Blackfan anaemia (DBA; n = 30), other rare anaemias (n = 22) or beta-thalassaemia (n = 85). Dosage was determined by baseline liver iron concentration (LIC).
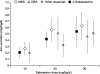
Results: In patients with baseline LIC > or = 7 mg Fe/g dry weight, deferasirox initiated at 20 or 30 mg/kg/d produced statistically significant decreases in LIC (P < 0.001); these decreases were greatest in MDS and least in DBA. As chelation efficiency and iron excretion did not differ significantly between disease groups, the differences in LIC changes are consistent with mean transfusional iron intake (least in MDS: 0.28 +/- 0.14 mg/kg/d; greatest in DBA: 0.4 +/- 0.11 mg/kg/d). Overall, LIC changes were dependent on dose (P < 0.001) and transfusional iron intake (P < 0.01), but not statistically different between disease groups. Changes in serum ferritin and LIC were correlated irrespective of disease group (r = 0.59), supporting the potential use of serum ferritin for monitoring deferasirox therapy. Deferasirox had a safety profile compatible with long-term use. There were no disease-specific safety/tolerability effects: the most common adverse events were gastrointestinal disturbances, skin rash and non-progressive serum creatinine increases.
Conclusions: Deferasirox is effective for reducing iron burden with a defined, clinically manageable safety profile in patients with various transfusion-dependent anaemias. There were no disease-specific adverse events. Once differences in transfusional iron intake are accounted for, dose-dependent changes in LIC or serum ferritin are similar in MDS and other disease groups.
Figures

References
-
- Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95:26–36. - PubMed
-
- Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–61. - PubMed
-
- Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–52. - PubMed
-
- Cazzola M, Malcovati L. Myelodysplastic syndromes—coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–8. - PubMed
-
- Malcovati L, Della Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

