Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2
- PMID: 18028534
- PMCID: PMC2211299
- DOI: 10.1186/1471-2121-8-49
Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2
Abstract
Background: Tight junctions are required for epithelial barrier formation and participate in the regulation of signalling mechanisms that control proliferation and differentiation. ZO-1 is a tight junction-associated adaptor protein that regulates gene expression, junction assembly and epithelial morphogenesis. We have previously demonstrated that the heat shock protein Apg-2 binds ZO-1 and thereby regulates its role in cell proliferation. Here, we addressed the question whether Apg-2 is also important for junction formation and epithelial morphogenesis.
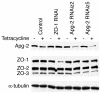
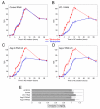
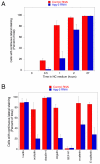
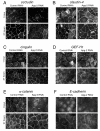
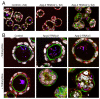
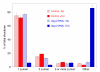
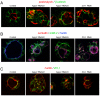
Results: We demonstrate that depletion of Apg-2 by RNAi in MDCK cells did not prevent formation of functional tight junctions. Similar to ZO-1, however, reduced expression of Apg-2 retarded de novo junction assembly if analysed in a Ca-switch model. Formation of functional junctions, as monitored by measuring transepithelial electrical resistance, and recruitment of tight and adherens junction markers were retarded. If cultured in three dimensional extracellular matrix gels, Apg-2 depleted cells, as previously shown for ZO-1 depleted cells, did not form hollow polarised cysts but poorly organised, irregular structures.
Conclusion: Our data indicate that Apg-2 regulates junction assembly and is required for normal epithelial morphogenesis in a three-dimensional culture system, suggesting that Apg-2 is an important regulator of epithelial differentiation. As the observed phenotypes are similar to those previously described for ZO-1 depleted cells and depletion of Apg-2 retards junctional recruitment of ZO-1, regulation of ZO-1 is likely to be an important functional role for Apg-2 during epithelial differentiation.
Figures


References
-
- Aijaz S, Balda MS, Matter K. Tight Junctions: Molecular Architecture and Function. Int Rev Cytol. 2006;248:261–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

