Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial
- PMID: 18029109
- PMCID: PMC2386758
- DOI: 10.1016/j.ijrobp.2007.08.041
Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial
Abstract
Purpose: To test whether the angiotensin-converting enzyme inhibitor captopril was effective in mitigating chronic renal failure after hematopoietic stem cell transplantation (HSCT).
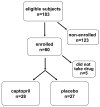
Methods and materials: A total of 55 subjects undergoing total body irradiation (TBI)-HSCT were enrolled in this randomized controlled trial. Captopril or identical placebo was started at engraftment and continued as tolerated until 1 year after HSCT.
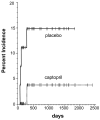
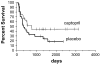
Results: The baseline serum creatinine and calculated glomerular filtration rate (GFR) did not differ between groups. The 1-year serum creatinine level was lower and the GFR higher in the captopril compared with the placebo group (p = 0.07 for GFR). Patient survival was higher in the captopril compared with the placebo group, but this was also not statistically significant (p = 0.09). In study subjects who received the study drug for more than 2 months, the 1-year calculated GFRs were 92 mL/min and 80 mL/min, for the captopril and placebo groups, respectively (p = 0.1). There was no adverse effect on hematologic outcome.
Conclusions: There is a trend in favor of captopril in mitigation of chronic renal failure after radiation-based HSCT.
Conflict of interest statement
Conflicts of interest
No conflicts of interest exist
Figures
References
-
- Cohen EP. Renal failure after bone marrow transplantation. Lancet. 2001;357:6–7. - PubMed
-
- Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–58. - PubMed
-
- Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease after bone marrow transplantation: poor survival compared to other causes of ESRD. Nephron. 1998;79:408–412. - PubMed
-
- Weiss AS, Sandmeier BM, Storer B, et al. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94. - PubMed
-
- Deconinck E, Kribs M, Rebibou JM, et al. Cytomegalovirus infection and chronic graft versus host disease are significant predictors of renal failure after allogeneic hematopoietic stem cell transplantation. Haematologia. 2005;90:569–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

