Assessment of the magnitude of growth hormone hypersecretion in active acromegaly: reliability of different sampling models
- PMID: 18029464
- PMCID: PMC2243233
- DOI: 10.1210/jc.2007-1451
Assessment of the magnitude of growth hormone hypersecretion in active acromegaly: reliability of different sampling models
Abstract
Context: The pulsatility of GH secretion in acromegaly poses difficulty in ascertaining true daily GH milieu in patients with this disease. Intensive GH sampling [every 10-20 (Q10-20) min for 24 h] is not practical in clinical practice.
Objective: Our objective was to ascertain reliability of abbreviated sampling protocols to reflect true 24-h mean GH concentrations in patients with acromegaly.
Design: An analysis of previously obtained plasma GH profiles was performed.
Setting: The analysis was performed at the General Clinical Research Center at the University of Michigan.
Patients: A total of 115 GH profiles obtained in 94 patients with active acromegaly were examined.
Intervention: Frequent blood sampling, i.e. Q10-20 min for 24 h, was performed.
Main outcome measures: Concordance of 24-h mean GH concentrations derived from Q10- to 20-min samplings with abbreviated GH sampling schedules was performed. The study was planned after data collection.
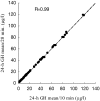
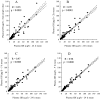
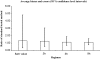
Results: All abbreviated schedules of GH sampling correlated well with the true 24-h plasma GH means (i.e. Q10- to 20-min sampling) (R = 0.93-0.98; P < 0.0001 for all). In the GH range more than 20 microg/liter, only 5 and 9-h means had R values more than 0.9. Single GH concentrations less than 1 microg/liter had a positive predictive value of only 0.29, and those with less than 2.5 microg/liter had a positive predictive value of 0.67 vs. their corresponding 24-h mean GH values of the same magnitude.
Conclusions: The intensity of GH sampling in patients with acromegaly may vary depending on the nature of the required information. Investigators and clinicians should be aware of the limitations of the abbreviated GH sampling protocols in acromegaly.
Figures
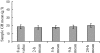


References
-
- Melmed S 2006 Medical progress: acromegaly. N Engl J Med 355:2558–2573 - PubMed
-
- Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, Ho K, Kleinberg D, Lamberts S, Laws E, Lombardi G, Vance ML, Werder KV, Wass J, Giustina A, Acromegaly Treatment Consensus Workshop Participants 2002 Guidelines for acromegaly management. J Clin Endocrinol Metab 87:4054–4058 - PubMed
-
- Clemmons DR 2006 Clinical utility of measurements of insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab 2:436–446 - PubMed
-
- Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL 2002 Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab 87:3537–3542 - PubMed
-
- Barkan AL, Beitins IZ, Kelch RP 1988 Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab 67:69–73 - PubMed

