Simple fluorescent sensors engineered with catalytic DNA 'MgZ' based on a non-classic allosteric design
- PMID: 18030352
- PMCID: PMC2077808
- DOI: 10.1371/journal.pone.0001224
Simple fluorescent sensors engineered with catalytic DNA 'MgZ' based on a non-classic allosteric design
Abstract
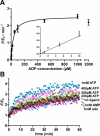
Most NAE (nucleic acid enzyme) sensors are composed of an RNA-cleaving catalytic motif and an aptameric receptor. They operate by activating or repressing the catalytic activity of a relevant NAE through the conformational change in the aptamer upon target binding. To transduce a molecular recognition event to a fluorescence signal, a fluorophore-quencher pair is attached to opposite ends of the RNA substrate such that when the NAE cleaves the substrate, an increased level of fluorescence can be generated. However, almost all NAE sensors to date harbor either NAEs that cannot accommodate a fluorophore-quencher pair near the cleavage site or those that can accept such a modification but require divalent transition metal ions for catalysis. Therefore, the signaling magnitude and the versatility of current NAE sensors might not suffice for analytical and biological applications. Here we report an RNA-cleaving DNA enzyme, termed 'MgZ', which depends on Mg(2+) for its activity and can accommodate bulky dye moieties next to the cleavage site. MgZ was created by in vitro selection. The selection scheme entailed acidic buffering and ethanol-based reaction stoppage to remove selfish DNAs. Characterization of MgZ revealed a three-way junction structure, a cleavage rate of 1 min(-1), and 26-fold fluorescence enhancement. Two ligand-responsive NAE sensors were rationally designed by linking an aptamer sequence to the substrate of MgZ. In the absence of the target, the aptamer-linked substrate is locked into a conformation that prohibits MgZ from accessing the substrate. In the presence of the target, the aptamer releases the substrate, which induces MgZ-mediated RNA cleavage. The discovery of MgZ and the introduction of the above NAE sensor design strategy should facilitate future efforts in sensor engineering.
Conflict of interest statement
Figures






References
-
- Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344:467–468. - PubMed
-
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. - PubMed
-
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

