Rapid CD4 decline after interruption of non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy in a resource-limited setting
- PMID: 18031583
- PMCID: PMC2211500
- DOI: 10.1186/1742-6405-4-26
Rapid CD4 decline after interruption of non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy in a resource-limited setting
Abstract
Background: Non-nucleoside reverse transcriptase inhibitor (NNRTI) with stavudine and lamivudine is widely used as the first-line antiretroviral therapy (ART) in resource-limited settings. Lipodystrophy is common and options for switching ART regimen are limited; this situation can lead to patients' poor adherence and antiretroviral resistance. Treatment interruption (TI) in patients with high CD4 cell counts, lipodystrophy, and limited options may be an alternative in resource-limited settings. This study aimed to determine time to resume ART after TI and predictors for early resumption of ART in a resource-limited setting.
Methods: A prospective study was conducted in January 2005 to December 2006 and enrolled HIV-infected patients with HIV-1 RNA <50 copies/mL, CD4 > 350 cells/mm3, and willing to interrupt ART. CD4 cell count, HIV-1 RNA, lipid profile, and lipodystrophy were assessed at baseline and every 3 months. ART was resumed when CD4 declined to <250 cells/mm3 or developed HIV-related symptoms. Patients were grouped based on ART regimens [NNRTI or protease inhibitor (PI)] prior to TI.
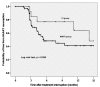
Results: There were 99 patients, 85 in NNRTI group and 14 in PI group. Mean age was 40.6 years; 46% were males. Median duration of ART was 47 months. Median nadir CD4 and baseline CD4 were 151 and 535 cells/mm3, respectively. Median CD4 change at 3 months after TI were -259 (NNRTI) and -105 (PI) cells/mm3 (p = 0.038). At 13-month median follow-up, there was no AIDS-defining illness; 38% (NNRTI) and 29% (PI) of patients developed HIV-related symptoms. ART was resumed in 51% (NNRTI) and 36% (PI) of patients (p = 0.022). By Kaplan-Meier analysis, median time to resume ART was 5.5 (NNRTI) and 14.2 (PI) months (log rank test, p = 0.026). By Cox's regression analysis, NNRTI-based ART (HR 4.9; 95%CI, 1.5-16.3), nadir CD4 <100 cells/mm3 (HR 2.7; 95%CI 1.4-5.3) and baseline CD4 <500 cells/mm3 (HR 1.6; 95%CI, 1.2-3.1) were predictors for early ART resumption.
Conclusion: TI of NNRTI-based ART leads to rapid CD4 decline and high probability of early ART resumption and should be avoided. It is necessary to scale-up the options for HIV-infected patients with lipodystrophy in resource-limited settings.
Figures
References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. - DOI - PubMed
-
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, Wainberg MA, Smith D, Robinson P, Hall D, Myers M, Lange JM. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS trial. Italy, The Netherlands, Canada and Australia study. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. - DOI - PubMed
-
- Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–46. doi: 10.1097/01.qai.0000230521.86964.86. - DOI - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK, 903 Study Group Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. - DOI - PubMed
-
- US FDA First of a kind in HIV treatment. FDA Consum. 2006;40:34. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous