Induction of particle polymorphism by cucumber necrosis virus coat protein mutants in vivo
- PMID: 18032493
- PMCID: PMC2224465
- DOI: 10.1128/JVI.01976-07
Induction of particle polymorphism by cucumber necrosis virus coat protein mutants in vivo
Abstract
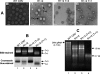
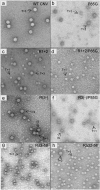
The Cucumber necrosis virus (CNV) particle is a T=3 icosahedron consisting of 180 identical coat protein (CP) subunits. Plants infected with wild-type CNV accumulate a high number of T=3 particles, but other particle forms have not been observed. Particle polymorphism in several T=3 icosahedral viruses has been observed in vitro following the removal of an extended N-terminal region of the CP subunit. In the case of CNV, we have recently described the structure of T=1 particles that accumulate in planta during infection by a CNV mutant (R1+2) in which a large portion of the N-terminal RNA binding domain (R-domain) has been deleted. In this report we further describe properties of this mutant and other CP mutants that produce polymorphic particles. The T=1 particles produced by R1+2 mutants were found to encapsidate a 1.9-kb RNA species as well as smaller RNA species that are similar to previously described CNV defective interfering RNAs. Other R-domain mutants were found to encapsidate a range of specifically sized less-than-full-length CNV RNAs. Mutation of a conserved proline residue in the arm domain near its junction with the shell domain also influenced T=1 particle formation. The proportion of polymorphic particles increased when the mutation was incorporated into R-domain deletion mutants. Our results suggest that both the R-domain and the arm play important roles in the formation of T=3 particles. In addition, the encapsidation of specific CNV RNA species by individual mutants indicates that the R-domain plays a role in the nature of CNV RNA encapsidated in particles.
Figures





References
-
- Annamalai, P., and A. L. Rao. 2005. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology 33896-111. - PubMed
-
- Ban, N., and A. McPherson. 1995. The structure of satellite panicum mosaic virus at 1.9 A resolution. Nat. Struct. Biol. 2882-890. - PubMed
-
- Basnayake, V. R., T. L. Sit, and S. A. Lommel. 2006. The genomic RNA packaging scheme of Red clover necrotic mosaic virus. Virology 345532-539. - PubMed
-
- Bergdoll, M., M. H. Remy, C. Cagnon, J. M. Masson, and P. Dumas. 1997. Proline-dependent oligomerization with arm exchange. Structure 5391-401. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

