Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine
- PMID: 18032593
- PMCID: PMC2238039
- DOI: 10.1128/CVI.00336-07
Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine
Abstract
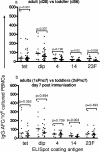
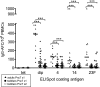
Glycoconjugate vaccines have dramatically reduced the incidence of encapsulated bacterial diseases in toddlers under 2 years of age, but vaccine-induced antibody levels in this age group wane rapidly. We immunized adults and 12-month-old toddlers with heptavalent pneumococcal conjugate vaccine to determine differences in B-cell and antibody responses. The adults and 12-month-old toddlers received a pneumococcal conjugate vaccine. The toddlers received a second dose at 14 months of age. The frequencies of diphtheria toxoid and serotype 4, 14, and 23F polysaccharide-specific plasma cells and memory B cells were determined by enzyme-linked immunospot assay. The toddlers had no preexisting polysaccharide-specific memory B cells or serum immunoglobulin G (IgG) antibody but had good diphtheria toxoid-specific memory responses. The frequencies of plasma cells and memory B cells increased by day 7 (P < 0.0001) in the adults and the toddlers following a single dose of conjugate, but the polysaccharide responses were significantly lower in the toddlers than in the adults (P = 0.009 to <0.001). IgM dominated the toddler antibody responses, and class switching to the IgG was serotype dependent. A second dose of vaccine enhanced the antibody and memory B-cell responses in the toddlers but not the ex vivo plasma cell responses. Two doses of pneumococcal conjugate vaccine are required in toddlers to generate memory B-cell frequencies and antibody class switching for each pneumococcal polysaccharide equivalent to that seen in adults.
Figures




References
-
- Abraham-Van Parijs, B. 2004. Review of pneumococcal conjugate vaccine in adults: implications on clinical development. Vaccine 22:1362-1371. - PubMed
-
- Anderson, E. L., D. J. Kennedy, K. M. Geldmacher, J. Donnelly, and P. M. Mendelman. 1996. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J. Pediatr. 128:649-653. - PubMed
-
- Barnett, E. D., S. I. Pelton, H. J. Cabral, R. D. Eavey, C. Allen, M. J. Cunningham, E. R. McNamara, and J. O. Klein. 1999. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin. Infect. Dis. 29:191-192. - PubMed
-
- Barzilay, E. J., K. L. O'Brien, Y. S. Kwok, R. M. Hoekstra, E. R. Zell, R. Reid, M. Santosham, C. G. Whitney, and D. R. Feikin. 2006. Could a single dose of pneumococcal conjugate vaccine in children be effective? Modeling the optimal age of vaccination. Vaccine 24:904-913. - PubMed
-
- Baxendale, H. E., Z. Davis, H. N. White, M. B. Spellerberg, F. K. Stevenson, and D. Goldblatt. 2000. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol. 30:1214-1223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

