Chronic stimulation of Nod2 mediates tolerance to bacterial products
- PMID: 18032608
- PMCID: PMC2148308
- DOI: 10.1073/pnas.0706097104
Chronic stimulation of Nod2 mediates tolerance to bacterial products
Abstract
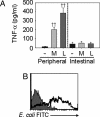
The Toll-like receptor (TLR) and nucleotide-binding oligomerization domain (Nod) families of proteins are critical for bacterial recognition, and, acutely, this frequently leads to proinflammatory responses. Polymorphisms in Nod2 (CARD 15) are associated with an increased likelihood of developing Crohn's disease. However, it is not yet clear how Nod2 dysfunctions lead to defects in human intestinal immune homeostasis. Studies to date have focused on functions after acute, rather than chronic, Nod2 stimulation. However, the intestine is an environment of chronic bacterial product exposure with tolerance to luminal flora. We therefore hypothesized that long-term Nod2 stimulation contributes to down-regulation of inflammatory responses from innate immune receptors. We found that pretreatment with muramyl dipeptide (MDP), a ligand for Nod2, significantly decreased production of the proinflammatory cytokines TNF-alpha, IL-8, and IL-1beta upon Nod2, TLR4, and TLR2 restimulation in primary human monocyte-derived macrophages from a large cohort of individuals. Importantly, TNF-alpha-induced production of proinflammatory cytokines remained intact in these same cells. MDP-stimulated macrophages from Crohn's disease-relevant Leu1007insC Nod2 homozygote individuals were deficient in their ability to cross-tolerize to subsequent treatment with TLR2 and TLR4 ligands. We show that acute Nod2 stimulation induced IRAK-1 activation, and that chronic MDP treatment down-regulated IRAK-1 activation upon Nod2 or TLR4 restimulation. In a subset of individuals, chronic Nod2 stimulation induced expression of the IRAK-1 inhibitory protein IRAK-M. Significantly, intestinal macrophages exhibit tolerance to MDP per production of inflammatory cytokines. These results illustrate a role for chronic stimulation of Nod2 in mediating tolerance to bacterial products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Podolsky DK. N Engl J Med. 2002;347:417–429. - PubMed
-
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. Nature. 2001;411:603–606. - PubMed
-
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Nature. 2001;411:599–603. - PubMed
-
- Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JP. Am J Gastroenterol. 2004;99:2393–2404. - PubMed
-
- Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. J Biol Chem. 2002;277:41701–41705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

