Cortex mediates multisensory but not unisensory integration in superior colliculus
- PMID: 18032649
- PMCID: PMC6673293
- DOI: 10.1523/JNEUROSCI.3524-07.2007
Cortex mediates multisensory but not unisensory integration in superior colliculus
Abstract
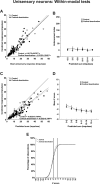
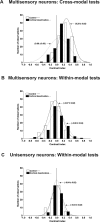
Converging cortical influences from the anterior ectosylvian sulcus and the rostral lateral suprasylvian sulcus were shown to have a multisensory-specific role in the integration of sensory information in superior colliculus (SC) neurons. These observations were based on changes induced by cryogenic deactivation of these cortico-SC projections. Thus, although the results indicated that they played a critical role in integrating SC responses to stimuli derived from different senses (i.e., visual-auditory), they played no role in synthesizing its responses to stimuli derived from within the same sense (visual-visual). This was evident even in the same multisensory neurons. The results suggest that very different neural circuits have evolved to code combinations of cross-modal and within-modal stimuli in the SC, and that the differences in multisensory and unisensory integration are likely caused by differences in the configuration of each neuron's functional inputs rather than to any inherent differences among the neurons themselves. The specificity of these descending influences was also apparent in the very different ways in which they affected responses to the component cross-modal stimuli and their actual integration. Furthermore, they appeared to target only multisensory neurons and not their unisensory neighbors.
Figures






Comment in
-
The influences of associative cortices on cross-modal integration in the superior colliculus.J Neurosci. 2008 Feb 20;28(8):1787-8. doi: 10.1523/JNEUROSCI.5368-07.2008. J Neurosci. 2008. PMID: 18287494 Free PMC article. Review. No abstract available.
References
-
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol. 2007;97:3193–3205. - PubMed
-
- Anastasio TJ, Patton PE, Belkacem-Boussaid K. Using Bayes' rule to model multisensory enhancement in the superior colliculus. Neural Comput. 2000;12:1165–1187. - PubMed
-
- Bolognini N, Frassinetti F, Serino A, Ladavas E. “Acoustical vision” of below threshold stimuli: interaction among spatially converging audiovisual inputs. Exp Brain Res. 2004;160:273–282. - PubMed
-
- Calvert GA, Spence C, Stein BE. The handbook of the multisensory processes. Cambridge, MA: MIT; 2004.
-
- Clemo HR, Stein BE. Effects of cooling somatosensory cortex on response properties of tactile cells in the superior colliculus. J Neurophysiol. 1986;55:1352–1368. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
