Central resistin induces hepatic insulin resistance via neuropeptide Y
- PMID: 18032666
- PMCID: PMC6673286
- DOI: 10.1523/JNEUROSCI.2443-07.2007
Central resistin induces hepatic insulin resistance via neuropeptide Y
Abstract
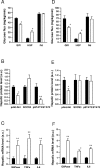
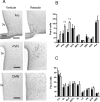
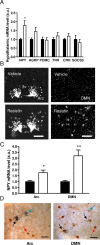
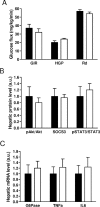
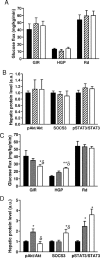
Sensing of peripheral hormones and nutrients by the hypothalamus plays an important role in maintaining peripheral glucose homeostasis. The hormone resistin impairs the response to insulin in liver and other peripheral tissues. Here we demonstrate that in normal mice resistin delivered in the lateral cerebral ventricle increased endogenous glucose production during hyperinsulinemic-euglycemic clamp, consistent with induction of hepatic insulin resistance. In agreement, central resistin inhibited Akt phosphorylation and increased the expression of glucose-6-phosphatase, the enzyme regulating glucose output in the liver. Central resistin induced expression of proinflammatory cytokines as well as suppressor of cytokine signaling-3, a negative regulator of insulin action in liver. Central infusion of resistin was associated with neuronal activation in the arcuate, paraventricular and dorsomedial nuclei, and increased neuropeptide Y (NPY) expression in the hypothalamus. The effects of central resistin on glucose production as well as hepatic expression of proinflammatory cytokines were abrogated in mice lacking NPY. Pretreatment of wild-type mice with antagonists of the NPY Y1 receptor, but not the Y5 receptor, also prevented the effects of central resistin. Together, these results suggest that resistin action on NPY neurons is an important regulator of hepatic insulin sensitivity.
Figures
References
-
- Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14(Suppl 5):242S–249S. - PubMed
-
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. - PubMed
-
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. - PubMed
-
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
