Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability
- PMID: 18032670
- PMCID: PMC6673301
- DOI: 10.1523/JNEUROSCI.4061-07.2007
Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability
Abstract
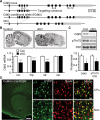
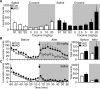
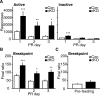
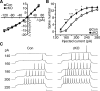
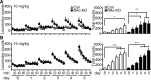
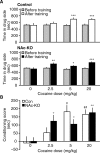
Cyclin-dependent kinase 5 (Cdk5) regulates dopamine neurotransmission and has been suggested to serve as a homeostatic target of chronic psychostimulant exposure. To study the role of Cdk5 in the modulation of the cellular and behavioral effects of psychoactive drugs of abuse, we developed Cre/loxP conditional knock-out systems that allow temporal and spatial control of Cdk5 expression in the adult brain. Here, we report the generation of Cdk5 conditional knock-out (cKO) mice using the alphaCaMKII promoter-driven Cre transgenic line (CaMKII-Cre). In this model system, loss of Cdk5 in the adult forebrain increased the psychomotor-activating effects of cocaine. Additionally, these CaMKII-Cre Cdk5 cKO mice show enhanced incentive motivation for food as assessed by instrumental responding on a progressive ratio schedule of reinforcement. Behavioral changes were accompanied by increased excitability of medium spiny neurons in the nucleus accumbens (NAc) in Cdk5 cKO mice. To study NAc-specific effects of Cdk5, another model system was used in which recombinant adeno-associated viruses expressing Cre recombinase caused restricted loss of Cdk5 in NAc neurons. Targeted knock-out of Cdk5 in the NAc facilitated cocaine-induced locomotor sensitization and conditioned place preference for cocaine. These results suggest that Cdk5 acts as a negative regulator of neuronal excitability in the NAc and that Cdk5 may govern the behavioral effects of cocaine and motivation for reinforcement.
Figures

References
-
- Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann NY Acad Sci. 2004;1025:335–344. - PubMed
-
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. - PubMed
-
- Bibb JA. Role of Cdk5 in neuronal signaling, plasticity, and drug abuse. Neurosignals. 2003;12:191–199. - PubMed
-
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. - PubMed
-
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
