Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning
- PMID: 18032673
- PMCID: PMC6673287
- DOI: 10.1523/JNEUROSCI.3373-07.2007
Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning
Abstract
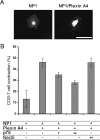
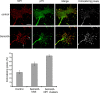
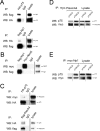
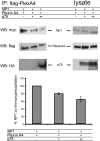
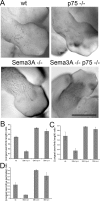
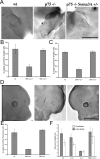
The p75 neurotrophin receptor (p75(NTR)) interacts with multiple ligands and coreceptors. It is thought to mediate myelin growth inhibition as part of the Nogo receptor complex, in addition to its other roles. Paradoxically, however, peripheral axons of p75(ExonIII-/-) mutant embryos are severely stunted. This inhibition of axon growth may be a result of neurite elongation defects in p75(NTR) mutant neurons. Here, we show that p75(ExonIII-/-) DRG neurons are hypersensitive to the repellent molecule Semaphorin3A (Sema3A). NGF modulates Sema3A activity equally well in both the p75(NTR) mutant and wild-type neurons, indicating that the hypersensitivity of p75(NTR) mutant neurons is probably not related to their NGF receptor activity. Neuropilin1 and p75(NTR) partially colocalize in DRG growth cones. After Sema3A stimulation, the degree of colocalization is dramatically increased, particularly in clusters associated with Sema3A receptor complex activation. Coimmunoprecipitation studies show that p75(NTR) interacts directly with the Sema3A receptors Neuropilin1 and PlexinA4. When coexpressed with both Neuropilin1 and PlexinA4, p75(NTR) reduces the interaction between these two receptor components. Finally, p75(NTR)/Sema3A double-mutant embryos show growth similar to that observed in Sema3A-null mice. These data indicate that p75(NTR) is an important functional modulator of Sema3A activity and that, in the absence of p75(NTR), oversensitivity to Sema3A leads to severe reduction in sensory innervation. Our results also suggest that while inhibition of p75(NTR) in CNS injury may enhance nerve regeneration resulting from the inhibition of myelin-associated protein, it may also inhibit nerve regeneration through its modulation of Sema3A.
Figures

References
-
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE. 2004;2004:pe24. - PubMed
-
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. - PubMed
-
- Ben-Zvi A, Yagil Z, Hagalili Y, Klein H, Lerman O, Behar O. Semaphorin 3A and neurotrophins: a balance between apoptosis and survival signaling in embryonic DRG neurons. J Neurochem. 2006;96:585–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
