Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy
- PMID: 18032674
- PMCID: PMC3087381
- DOI: 10.1523/JNEUROSCI.3605-07.2007
Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy
Abstract
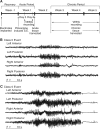
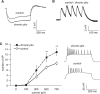
Ion channelopathy plays an important role in human epilepsy with a genetic cause and has been hypothesized to occur in epilepsy after acquired insults to the CNS as well. Acquired alterations of ion channel function occur after induction of status epilepticus (SE) in animal models of epilepsy, but it is unclear how they correlate with the onset of spontaneous seizures. We examined the properties of hyperpolarization-activated cation (HCN) channels in CA1 hippocampal pyramidal neurons in conjunction with video-EEG (VEEG) recordings to monitor the development of spontaneous seizures in the rat pilocarpine model of epilepsy. Our results showed that dendritic HCN channels were significantly downregulated at an acute time point 1 week postpilocarpine, with loss of channel expression and hyperpolarization of voltage-dependent activation. This downregulation progressively increased when epilepsy was established in the chronic period. Surprisingly, VEEG recordings during the acute period showed that a substantial fraction of animals were already experiencing recurrent seizures. Suppression of these seizures with phenobarbital reversed the change in the voltage dependence of I(h), the current produced by HCN channels, but did not affect the loss of HCN channel expression. These results suggest two mechanisms of HCN channel downregulation after SE, one dependent on and one independent of recurrent seizures. This early and progressive downregulation of dendritic HCN channel function increases neuronal excitability and may be associated with both the process of epileptogenesis and maintenance of the epileptic state.
Figures




References
-
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. - PubMed
-
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. - PubMed
-
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
