Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals
- PMID: 18032677
- PMCID: PMC4313565
- DOI: 10.1523/JNEUROSCI.4290-06.2007
Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals
Abstract
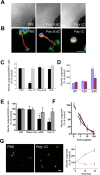
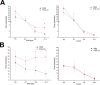
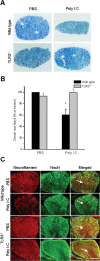
Toll is a cell surface receptor with well described roles in the developmental patterning of invertebrates and innate immunity in adult Drosophila. Mammalian toll-like receptors represent a family of Toll orthologs that function in innate immunity by recognizing molecular motifs unique to pathogens or injured tissue. One member in this family of pattern recognition receptors, toll-like receptor 3 (TLR3), recognizes viral double-stranded RNA and host mRNA. We examined the expression and function of TLRs in the nervous system and found that TLR3 is expressed in the mouse central and peripheral nervous systems and is concentrated in the growth cones of neurons. Activation of TLR3 by the synthetic ligand polyinosine:polycytidylic acid (poly I:C) or by mRNA rapidly causes growth cone collapse and irreversibly inhibits neurite extension independent of nuclear factor kappaB. Mice lacking functional TLR3 were resistant to the neurodegenerative effects of poly I:C. Neonatal mice injected with poly I:C were found to have fewer axons exiting dorsal root ganglia and displayed related sensorimotor deficits. No effect of poly I:C was observed in mice lacking functional TLR3. Together, these findings provide evidence that an innate immune pattern recognition receptor functions autonomously in neurons to regulate axonal growth and advances a novel hypothesis that this class of receptors may contribute to injury and limited CNS regeneration.
Figures




References
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Andaloussi AE, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54:526–535. - PubMed
-
- Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985a;42:779–789. - PubMed
-
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985b;42:791–798. - PubMed
-
- Bergmann-Leitner ES, Leitner WW. Danger, death and DNA vaccines. Microbes Infect. 2004;6:319–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
