Differential intracellular distribution of DNA complexed with polyethylenimine (PEI) and PEI-polyarginine PTD influences exogenous gene expression within live COS-7 cells
- PMID: 18036259
- PMCID: PMC2211466
- DOI: 10.1186/1479-0556-5-11
Differential intracellular distribution of DNA complexed with polyethylenimine (PEI) and PEI-polyarginine PTD influences exogenous gene expression within live COS-7 cells
Abstract
Background: Polyethylenimine (PEI) is one of the most efficient and versatile non-viral vectors available for gene delivery. Despite many advantages over viral vectors, PEI is still limited by lower transfection efficiency compared to its viral counterparts. Considerable investigation is devoted to the modification of PEI to incorporate virus-like properties to improve its efficacy, including the incorporation of the protein transduction domain (PTD) polyarginine (Arg); itself demonstrated to facilitate membrane translocation of molecular cargo. There is, however, limited understanding of the underlying mechanisms of gene delivery facilitated by both PEI and PEI-bioconjugates such as PEI-polyarginine (PEI-Arg) within live cells, which once elucidated will provide valuable insights into the development of more efficient non-viral gene delivery vectors.
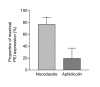
Methods: PEI and PEI-Arg were investigated for their ability to facilitate DNA internalization and gene expression within live COS-7 cells, in terms of the percentage of cells transfected and the relative amount of gene expression per cell. Intracellular trafficking of vectors was investigated using fluorescent microscopy during the first 5 h post transfection. Finally, nocodazole and aphidicolin were used to investigate the role of microtubules and mitosis, respectively, and their impact on PEI and PEI-Arg mediated gene delivery and expression.
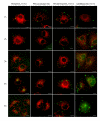
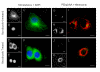
Results: PEI-Arg maintained a high cellular DNA uptake efficiency, and facilitated as much as 2-fold more DNA internalization compared to PEI alone. PEI, but not PEI-Arg, displayed microtubule-facilitated trafficking, and was found to accumulate within close proximity to the nucleus. Only PEI facilitated significant gene expression, whereas PEI-Arg conferred negligible expression. Finally, while not exclusively dependent, microtubule trafficking and, to a greater extent, mitotic events significantly contributed to PEI facilitated gene expression.
Conclusion: PEI polyplexes are trafficked by an indirect association with microtubules, following endosomal entrapment. PEI facilitated expression is significantly influenced by a mitotic event, which is increased by microtubule organization center (MTOC)-associated localization of PEI polyplexes. PEI-Arg, although enhancing DNA internalization per cell, did not improve gene expression, highlighting the importance of microtubule trafficking for PEI vectors and the impact of the Arg peptide to intracellular trafficking. This study emphasizes the importance of a holistic approach to investigate the mechanisms of novel gene delivery vectors.
Figures
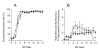

References
-
- Guillem VM, Aliño SF. Transfection pathways of nonspecific and targeted PEI-polyplexes. Gene Therapy and Molecular Biology. 2004;8:369–384.
LinkOut - more resources
Full Text Sources
Other Literature Sources

