Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice
- PMID: 18036594
- PMCID: PMC2189558
- DOI: 10.1053/j.gastro.2007.10.030
Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice
Abstract
Background & aims: Extracellular nucleotides are released from injured cells and bind purinergic-type 2 receptors (P2-Rs) that modulate inflammatory responses. Ectonucleotidases, such as CD39/nucleoside triphosphate diphosphohydrolase-1, hydrolyze extracellular nucleotides to integrate purinergic signaling responses. Because the role of extracellular nucleotides and CD39 in mediating inflammation and fibrosis are understood poorly, we studied the impact of CD39 gene deletion in a model of pancreatic disease.
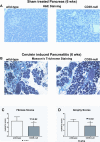
Methods: Pancreatitis was induced by cyclosporine pretreatment, followed by cerulein injections (50 mug/kg, 6 intraperitoneal injections/day, 3 times/wk); mice were killed at day 2, week 3, and week 6. Experimental parameters were correlated with cytokine levels in blood, RNA, and protein expression of purinergic and fibrosis markers in tissues. Immunohistochemistry and pancreatic morphometry of fibrosis were performed in wild-type and CD39-null mice. Effects of CD39 deletion on proliferation of primary pancreatic stellate cells (PSCs) were investigated in vitro.
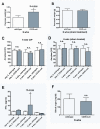
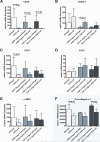
Results: Wild-type mice developed morphologic features of pancreatitis with the anticipated development of parenchymal atrophy and fibrosis. CD39 and P2-R became overexpressed in vascular and adventitious wild-type tissues. In contrast, CD39-null mice had inflammatory reactions but developed only minor pancreatic atrophy and limited fibrosis. Interferon-gamma became significantly increased in tissues and plasma of CD39-null mice. Wild-type PSCs expressed high levels of CD39 and P2-R. CD39-null PSCs showed decreased rates of proliferation and the expression of procollagen-alpha1 was inhibited significantly in vitro (P < .03).
Conclusions: CD39 deletion decreases fibrogenesis in experimental pancreatitis. Our data implicate extracellular nucleotides as modulators of PSC proliferation and collagen production in pancreatitis.
Figures




References
-
- Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. - PubMed
-
- Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grunert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. - PubMed
-
- Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–94. - PubMed
-
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

