Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB
- PMID: 18037434
- PMCID: PMC2277468
- DOI: 10.1016/j.jmb.2007.10.006
Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB
Abstract
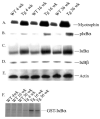
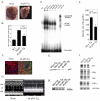
Activation of the nuclear factor (NF)-kappaB signaling pathway may be associated with the development of cardiac hypertrophy and its transition to heart failure (HF). The transgenic Myo-Tg mouse develops hypertrophy and HF as a result of overexpression of myotrophin in the heart associated with an elevated level of NF-kappaB activity. Using this mouse model and an NF-kappaB-targeted gene array, we first determined the components of NF-kappaB signaling cascade and the NF-kappaB-linked genes that are expressed during the progression to cardiac hypertrophy and HF. Second, we explored the effects of inhibition of NF-kappaB signaling events by using a gene knockdown approach: RNA interference through delivery of a short hairpin RNA against NF-kappaB p65 using a lentiviral vector (L-sh-p65). When the short hairpin RNA was delivered directly into the hearts of 10-week-old Myo-Tg mice, there was a significant regression of cardiac hypertrophy, associated with a significant reduction in NF-kappaB activation and atrial natriuretic factor expression. Our data suggest, for the first time, that inhibition of NF-kappaB using direct gene delivery of sh-p65 RNA results in regression of cardiac hypertrophy. These data validate NF-kappaB as a therapeutic target to prevent hypertrophy/HF.
Figures



References
-
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. - PubMed
-
- Kent RL, Mann DL, Cooper G. Signals for cardiac muscle hypertrophy in hypertension. J Cardiovasc. Pharmacol. 1991;17(Suppl 2):S7–13. - PubMed
-
- Baldwin AS., Jr. The NF-κB and I-κB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
-
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. - PubMed
-
- Chen F, Castranova V, Shi X, Demers LMA. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999;45:7–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

