Antithrombin III in critically ill patients: systematic review with meta-analysis and trial sequential analysis
- PMID: 18037615
- PMCID: PMC2137061
- DOI: 10.1136/bmj.39398.682500.25
Antithrombin III in critically ill patients: systematic review with meta-analysis and trial sequential analysis
Abstract
Objective: To evaluate the benefits and harms of antithrombin III in critically ill patients.
Design: Systematic review and meta-analysis of randomised trials.
Data sources: CENTRAL, Medline, Embase, International Web of Science, LILACS, the Chinese Biomedical Literature Database, and CINHAL (to November 2006); hand search of reference lists, contact with authors and experts, and search of registers of ongoing trials.
Review methods: Two reviewers independently selected parallel group randomised clinical trials comparing antithrombin with placebo or no intervention and extracted data related to study methods, interventions, outcomes, bias risk, and adverse events. Disagreements were resolved by discussion. Trials in any type of critically ill patients in intensive care were eligible. All trials, irrespective of blinding or language status, that compared any antithrombin III regimen with no intervention or placebo were included. Trials were considered to be at low risk of bias if they had adequate randomisation procedure, blinding, and used intention to treat analysis. Risk ratios with 95% confidence intervals were estimated with fixed and random effects models according to heterogeneity.
Main outcome measures: Mortality, length of stay in intensive care or hospital, quality of life, severity of sepsis, respiratory failure, duration of mechanical ventilation, incidence of surgical intervention, intervention effect among various populations, and adverse events (such as bleeding).
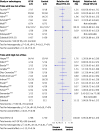
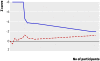
Results: 20 trials randomly assigning 3458 patients met inclusion criteria. Eight trials had low risk of bias. Compared with placebo or no intervention, antithrombin III did not reduce overall mortality (relative risk 0.96, 95% confidence interval 0.89 to 1.03). No subgroup analyses on risk of bias, populations of patients, or with and without adjuvant heparin yielded significant results. Antithrombin III increased the risk of bleeding events (1.52, 1.30 to 1.78). Heterogeneity was observed in only a few analyses.
Conclusion: Antithrombin III cannot be recommended for critically ill patients based on the available evidence.
Conflict of interest statement
Competing interests: None declared.
Figures






Comment in
-
Antithrombin III in critically ill patients.BMJ. 2007 Dec 15;335(7632):1219-20. doi: 10.1136/bmj.39399.552245.80. Epub 2007 Nov 23. BMJ. 2007. PMID: 18037614 Free PMC article.
References
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al, Recombinant human protein C worldwide evaluation in severe sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699-709. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. - PubMed
-
- Periti P. Current treatment of sepsis and endotoxaemia. Expert Opin Pharmacother 2000;1:1203-17. - PubMed
-
- Levi M, de Jonge E, van der Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann Med 2004;36:41-9. - PubMed
-
- Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Crit Care Med 2002;30(5 suppl):S325-31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
