Nicotinic acid: an old drug with a promising future
- PMID: 18037924
- PMCID: PMC2268066
- DOI: 10.1038/sj.bjp.0707528
Nicotinic acid: an old drug with a promising future
Abstract
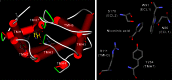
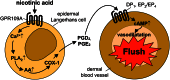
Nicotinic acid has been used for decades to treat dyslipidaemic states. In particular its ability to raise the plasma HDL cholesterol concentration has led to an increased interest in its pharmacological potential. The clinical use of nicotinic acid is somewhat limited due to several harmless but unpleasant side effects, most notably a cutaneous flushing phenomenon. With the recent discovery of a nicotinic acid receptor, it has become possible to better understand the mechanisms underlying the metabolic and vascular effects of nicotinic acid. Based on these new insights into the action of nicotinic acid, novel strategies are currently under development to maximize the pharmacological potential of this drug. The generation of both flush-reducing co-medications of nicotinic acid and novel drugs targeting the nicotinic acid receptor will provide future therapeutic options for the treatment of dyslipidaemic disorders.
Figures


References
-
- Aktories K, Jakobs KH, Schultz G. Nicotinic acid inhibits adipocyte adenylate cyclase in a hormone—like manner. FEBS Lett. 1980;115:11–14. - PubMed
-
- Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54:558–559. - PubMed
-
- Andersson RG, Aberg G, Brattsand R, Ericsson E, Lundholm L. Studies on the mechanism of flush induced by nicotinic acid. Acta Pharmacol Toxicol (Copenh) 1977;41:1–10. - PubMed
-
- Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19:151–157. - PubMed
-
- Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006;70:1844–1849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

