Alzheimer disease models and human neuropathology: similarities and differences
- PMID: 18038275
- PMCID: PMC2100431
- DOI: 10.1007/s00401-007-0312-8
Alzheimer disease models and human neuropathology: similarities and differences
Abstract
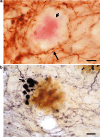
Animal models aim to replicate the symptoms, the lesions or the cause(s) of Alzheimer disease. Numerous mouse transgenic lines have now succeeded in partially reproducing its lesions: the extracellular deposits of Abeta peptide and the intracellular accumulation of tau protein. Mutated human APP transgenes result in the deposition of Abeta peptide, similar but not identical to the Abeta peptide of human senile plaque. Amyloid angiopathy is common. Besides the deposition of Abeta, axon dystrophy and alteration of dendrites have been observed. All of the mutations cause an increase in Abeta 42 levels, except for the Arctic mutation, which alters the Abeta sequence itself. Overexpressing wild-type APP alone (as in the murine models of human trisomy 21) causes no Abeta deposition in most mouse lines. Doubly (APP x mutated PS1) transgenic mice develop the lesions earlier. Transgenic mice in which BACE1 has been knocked out or overexpressed have been produced, as well as lines with altered expression of neprilysin, the main degrading enzyme of Abeta. The APP transgenic mice have raised new questions concerning the mechanisms of neuronal loss, the accumulation of Abeta in the cell body of the neurons, inflammation and gliosis, and the dendritic alterations. They have allowed some insight to be gained into the kinetics of the changes. The connection between the symptoms, the lesions and the increase in Abeta oligomers has been found to be difficult to unravel. Neurofibrillary tangles are only found in mouse lines that overexpress mutated tau or human tau on a murine tau -/- background. A triply transgenic model (mutated APP, PS1 and tau) recapitulates the alterations seen in AD but its physiological relevance may be discussed. A number of modulators of Abeta or of tau accumulation have been tested. A transgenic model may be analyzed at three levels at least (symptoms, lesions, cause of the disease), and a reading key is proposed to summarize this analysis.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC6725122', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC6725122/'}, {'type': 'PubMed', 'value': '15858047', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15858047/'}]}
- Adlard P, Perreau V, Pop V, Cotman CW (2005) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci Res 25:4217–4221 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC3887148', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC3887148/'}, {'type': 'PubMed', 'value': '10858586', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10858586/'}]}
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC6758022', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC6758022/'}, {'type': 'PubMed', 'value': '12417659', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12417659/'}]}
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22:9340–9351 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '7550356', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7550356/'}]}
- Alzheimer’s disease collaborative group, Clark RP, Hutton M, Fuldner RA, Froelich S, Karran B, Talbot C, Crook R, Lendon C, Prihar G, He C, Korenblat K, Martinez A, Wragg M, Busfield F, Behrens MI, Myers A, Norton J, Morris J, Mehta N, Pearson C, Lincoln S, Baker M, Duff K, Zehr C, Perez-Tur J, Houlden H, Ruiz A, Ossa J, Lopera F, Arcos M, Madrigal M, Collinge J, Humphreys C, Ashworth T, Sarner S, Fox N, Harvey R, Kennedy A, Roques P, Cline RT, Philips L, Venter JC, Forsell L, Axelman K, Lilius L, Johnston J, Cowburn R, Vitanen N, Winblad D, Kosik K, Haltia M, Poyhonen M, Dickson D, Mann D, Neary D, Snowden J, Lantos P, Lannfeld L, Rossor M, Roberts GW, Adams MD, Hardy J, Goate A (1995) The structure of the Presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat Genet 11:219–222 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC1698805', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1698805/'}, {'type': 'PubMed', 'value': '16877355', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16877355/'}]}
- Ambree O, Leimer U, Herring A, Gortz N, Sachser N, Heneka MT, Paulus W, Keyvani K (2006) Reduction of amyloid angiopathy and Abeta plaque burden after enriched housing in TgCRND8 mice: involvement of multiple pathways. Am J Pathol 169:544–552 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

