Developmental stage of oligodendrocytes determines their response to activated microglia in vitro
- PMID: 18039385
- PMCID: PMC2214724
- DOI: 10.1186/1742-2094-4-28
Developmental stage of oligodendrocytes determines their response to activated microglia in vitro
Abstract
Background: Oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes are both lost in central nervous system injury and disease. Activated microglia may play a role in OPC and oligodendrocyte loss or replacement, but it is not clear how the responses of OPCs and oligodendrocytes to activated microglia differ.
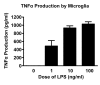
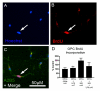
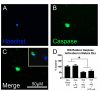
Methods: OPCs and microglia were isolated from rat cortex. OPCs were induced to differentiate into oligodendrocytes with thyroid hormone in defined medium. For selected experiments, microglia were added to OPC or oligodendrocyte cultures. Lipopolysaccharide was used to activate microglia and microglial activation was confirmed by TNFalpha ELISA. Cell survival was assessed with immunocytochemistry and cell counts. OPC proliferation and oligodendrocyte apoptosis were also assessed.
Results: OPCs and oligodendrocytes displayed phenotypes representative of immature and mature oligodendrocytes, respectively. Activated microglia reduced OPC survival, but increased survival and reduced apoptosis of mature oligodendrocytes. Activated microglia also underwent cell death themselves.
Conclusion: Activated microglia may have divergent effects on OPCs and mature oligodendrocytes, reducing OPC survival and increasing mature oligodendrocyte survival. This may be of importance because activated microglia are present in several disease states where both OPCs and mature oligodendrocytes are also reacting to injury. Activated microglia may simultaneously have deleterious and helpful effects on different cells after central nervous system injury.
Figures



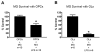
References
-
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. - PubMed
-
- Sun F, Lin C, McTigue DM, Shan X, C.A. T, J.C. B, Beattie MS. Dorsal rhizotomy induces oligodendrocyte progenitor proliferation and oligodendrocyte genesis, while spinal contusion induces oligodendrocyte death. J Neurosci. 2006.

