Modulation of pulmonary dendritic cell function during mycobacterial infection
- PMID: 18039834
- PMCID: PMC2223454
- DOI: 10.1128/IAI.01079-07
Modulation of pulmonary dendritic cell function during mycobacterial infection
Abstract
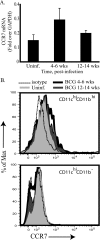
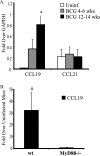
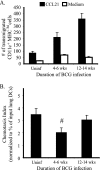
We have previously reported that during mycobacterial infection, naïve CD4(+) T-cell activation is enhanced in the lungs. We investigated the role of chemokine receptor CCR7 and its ligands in the ability of CD11c(+) lung dendritic cells (DCs) to activate naïve CD4(+) T cells during pulmonary infection with Mycobacterium bovis bacillus Calmette-Guérin (BCG). BCG infection resulted in the accumulation and maturation in the lungs of DCs that persisted as the mycobacterial burden declined. Lung DCs from infected mice expressed more major histocompatibility complex class II (MHC-II) than those from uninfected mice. CCR7 expression levels on lung DCs were comparable among uninfected and infected mice. The gene expression of the CCR7 ligand CCL19 progressively increased throughout BCG infection, and its expression was MyD88 dependent. CD11c(+) lung cells from BCG-infected mice activated ovalbumin (OVA)-specific naïve CD4(+) T cells more than CD11c(+) lung cells from uninfected mice. Interestingly, during peak mycobacterial infection, CD11c(hi) MHC(hi) lung DCs had slightly decreased chemotaxis toward the CCR7 ligand CCL21 and less efficiency in activating naive CD4(+) T cells than DCs from mice during late-stage infection, when few bacilli are found in the lung. These findings suggest that during BCG infection, the inflammation and sustained expression of CCL19 result in the recruitment, activation, and retention in the lung of DCs that can activate naïve CD4(+) T cells in situ.
Figures
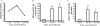

References
-
- Aloisi, F., and R. Pujol-Borrell. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6205-217. - PubMed
-
- Bardi, G., M. Lipp, M. Baggiolini, and P. Loetscher. 2001. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 313291-3297. - PubMed
-
- Bhatt, K., S. P. Hickman, and P. Salgame. 2004. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J. Immunol. 1722748-2751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

