Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence
- PMID: 18039940
- PMCID: PMC2224146
- DOI: 10.1128/EC.00370-07
Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence
Abstract
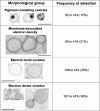
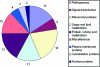
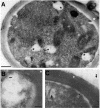
Cryptococcus neoformans produces vesicles containing its major virulence factor, the capsular polysaccharide glucuronoxylomannan (GXM). These vesicles cross the cell wall to reach the extracellular space, where the polysaccharide is supposedly used for capsule growth or delivered into host tissues. In the present study, we characterized vesicle morphology and protein composition by a combination of techniques including electron microscopy, proteomics, enzymatic activity, and serological reactivity. Secretory vesicles in C. neoformans appear to be correlated with exosome-like compartments derived from multivesicular bodies. Extracellular vesicles manifested various sizes and morphologies, including electron-lucid membrane bodies and electron-dense vesicles. Seventy-six proteins were identified by proteomic analysis, including several related to virulence and protection against oxidative stress. Biochemical tests indicated laccase and urease activities in vesicles. In addition, different vesicle proteins were recognized by sera from patients with cryptococcosis. These results reveal an efficient and general mechanism of secretion of pathogenesis-related molecules in C. neoformans, suggesting that extracellular vesicles function as "virulence bags" that deliver a concentrated payload of fungal products to host effector cells and tissues.
Figures


References
-
- Aoki, N., S. Jin-no, Y. Nakagawa, N. Asai, E. Arakawa, N. Tamura, T. Tamura, and T. Matsuda. 2007. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology 1483850-3862. - PubMed
-
- Barbosa, M. S., S. N. Baó, P. F. Andreotti, F. P. de Faria, M. S. S. Felipe, L. dos Santos Feitosa, M. J. S. Mendes-Giannini, and C. M. d. A. Soares. 2006. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 74382-389. - PMC - PubMed
-
- Batista, W. L., A. L. Matsuo, L. Ganiko, T. F. Barros, T. R. Veiga, E. Freymüller, and R. Puccia. 2006. The PbMDJ1 gene belongs to a conserved MDJ1/LON locus in thermodimorphic pathogenic fungi and encodes a heat shock protein that localizes to both the mitochondria and cell wall of Paracoccidioides brasiliensis. Eukaryot. Cell 5379-390. - PMC - PubMed
-
- Bicanic, T., and T. S. Harrison. 2004. Cryptococcal meningitis. Br. Med. Bull. 7299-118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G12 RR008124/RR/NCRR NIH HHS/United States
- R01 AI033774/AI/NIAID NIH HHS/United States
- R01 AI056070/AI/NIAID NIH HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- HL059842/HL/NHLBI NIH HHS/United States
- R01 AI052733/AI/NIAID NIH HHS/United States
- AI052733/AI/NIAID NIH HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- AI056070-01A2/AI/NIAID NIH HHS/United States
- 5G12RR008124/RR/NCRR NIH HHS/United States
- R01 AI033142/AI/NIAID NIH HHS/United States
- AI52733/AI/NIAID NIH HHS/United States
- AI033142/AI/NIAID NIH HHS/United States
- AI033774/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

