Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses
- PMID: 18039949
- PMCID: PMC2150965
- DOI: 10.1084/jem.20071094
Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses
Erratum in
- J Exp Med. 2008 Aug 4;205(8):1941. Suárez Fariñas, Mayte [corrected to Suárez-Fariñas, Mayte]
Abstract
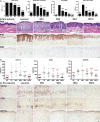
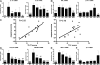
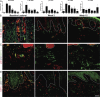
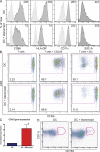
Biological agents have dramatically improved treatment options for patients with severe psoriasis. Etanercept (tumor necrosis factor [TNF] receptor-immunoglobulin fusion protein) is an effective treatment for many psoriasis patients, and blockade of TNF is considered to be its primary action. However, in this clinical trial, we show that etanercept has early inhibitory effects on a newly appreciated type of T cells: T helper type 17 (Th17) cells. Etanercept reduced the inflammatory dendritic cell products that drive Th17 cell proliferation (interleukin [IL] 23), as well as Th17 cell products and downstream effector molecules (IL-17, IL-22, CC chemokine ligand 20, and beta-defensin 4). In contrast, Th1 cellular products and effector molecules (interferon gamma, lymphotoxin alpha, and myxovirus resistance 1) were reduced late in disease resolution. This study suggests a role for Th17 in addition to Th1 cells in the pathogenesis of psoriasis. Th17 cells may be particularly important in driving epidermal activation in psoriatic plaques, whereas Th1 cells must also be eliminated for final disease resolution.
Figures

References
-
- Lowes, M.A., A.M. Bowcock, and J.G. Krueger. 2007. Pathogenesis and therapy of psoriasis. Nature. 445:866–873. - PubMed
-
- Blauvelt, A. 2007. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-β1. Expert Rev. Dermatol. 2:69–78.
-
- Prinz, J.C., B. Grob, S. Vollmer, P. Trommler, I. Strobel, M. Meurer, and G. Plewig. 1994. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur. J. Immunol. 24:593–598. - PubMed
-
- Bettelli, E., M. Oukka, and V.K. Kuchroo. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

