NSOM/QD-based nanoscale immunofluorescence imaging of antigen-specific T-cell receptor responses during an in vivo clonal Vγ2Vδ2 T-cell expansion
- PMID: 18039956
- PMCID: PMC2288727
- DOI: 10.1182/blood-2007-07-101691
NSOM/QD-based nanoscale immunofluorescence imaging of antigen-specific T-cell receptor responses during an in vivo clonal Vγ2Vδ2 T-cell expansion
Abstract
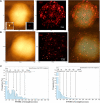
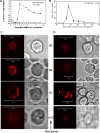
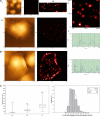
Nanoscale imaging of an in vivo antigen-specific T-cell immune response has not been reported. Here, the combined near-field scanning optical microscopy- and fluorescent quantum dot-based nanotechnology was used to perform immunofluorescence imaging of antigen-specific T-cell receptor (TCR) response in an in vivo model of clonal T-cell expansion. The near-field scanning optical microscopy/quantum dot system provided a best-optical-resolution (<50 nm) nano-scale imaging of Vgamma2Vdelta2 TCR on the membrane of nonstimulated Vgamma2Vdelta2 T cells. Before Ag-induced clonal expansion, these nonstimulating Vgamma2Vdelta2 TCRs appeared to be distributed differently from their alphabeta TCR counterparts on the cell surface. Surprisingly, Vgamma2Vdelta2 TCR nanoclusters not only were formed but also sustained on the membrane during an in vivo clonal expansion of Vgamma2Vdelta2 T cells after phosphoantigen treatment or phosphoantigen plus mycobacterial infection. The TCR nanoclusters could array to form nanodomains or microdomains on the membrane of clonally expanded Vgamma2Vdelta2 T cells. Interestingly, expanded Vgamma2Vdelta2 T cells bearing TCR nanoclusters or nanodomains were able to rerecognize phosphoantigen and to exert better effector function. These studies provided nanoscale insight into the in vivo T-cell immune response.
Figures




References
-
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

