Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis
- PMID: 18040043
- PMCID: PMC2148320
- DOI: 10.1073/pnas.0709443104
Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis
Abstract
Elevated expression of members of the BCL-2 pro-survival family of proteins can confer resistance to apoptosis in cancer cells. Small molecule obatoclax (GX15-070), which is predicted to occupy a hydrophobic pocket within the BH3 binding groove of BCL-2, antagonizes these members and induces apoptosis, dependent on BAX and BAK. Reconstitution in yeast confirmed that obatoclax acts on the pathway and overcomes BCL-2-, BCL-XL-, BCL-w-, and MCL-1-mediated resistance to BAX or BAK. The compound potently interfered with the direct interaction between MCL-1 and BAK in intact mitochondrial outer membrane and inhibited the association between MCL-1 and BAK in intact cells. MCL-1 has been shown to confer resistance to the BCL-2/BCL-XL/BCL-w-selective antagonist ABT-737 and to the proteasome inhibitor bortezomib. In both cases, this resistance was overcome by obatoclax. These findings support a rational clinical development opportunity for the compound in cancer indications or treatments where MCL-1 contributes to resistance to cell killing.
Conflict of interest statement
Conflict of interest statement: R.C.M., A.R., M.W., L.S., S.R.M.M., D.G., J.V., L.B., X.B., P.B., and G.C.S. are or were employees of Gemin X Biotechnologies, Inc.
Figures
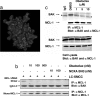


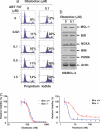
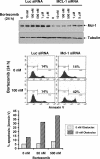
References
-
- Cory S, Vaux DL, Strasser A, Harris AW, Adams JM. Cancer Res. 1999;59(Suppl 7):1685s–1692s. - PubMed
-
- Korsmeyer SJ. Cancer Res. 1999;59(Suppl 7):1693s–1700s. - PubMed
-
- Danial NN, Korsmeyer SJ. Cell. 2004;116:205–219. - PubMed
-
- Vaux DL, Cory S, Adams JM. Nature. 1988;335:440–442. - PubMed
-
- Reed JC, Cuddy M, Slabiak T, Croce CM, Nowell PC. Nature. 1988;336:259–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials

