Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function
- PMID: 18040044
- PMCID: PMC2148310
- DOI: 10.1073/pnas.0709264104
Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function
Abstract
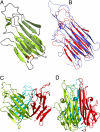
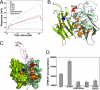
Glucocorticoid-induced TNF receptor ligand (GITRL), a recently identified member of the TNF family, binds to its receptor GITR on both effector and regulatory T cells and generates positive costimulatory signals implicated in a wide range of T cell functions. Structural analysis reveals that the human GITRL (hGITRL) ectodomain self-assembles into an atypical expanded homotrimer with sparse monomer-monomer interfaces. Consistent with the small intersubunit interfaces, hGITRL exhibits a relatively weak tendency to trimerize in solution and displays a monomer-trimer equilibrium not reported for other TNF family members. This unique assembly behavior has direct implications for hGITRL-GITR signaling, because enforced trimerization of soluble hGITRL ectodomain results in an approximately 100-fold increase in its receptor binding affinity and also in enhanced costimulatory activity. The apparent reduction in affinity that is the consequence of this dynamic equilibrium may represent a mechanism to realize the biologically optimal level of signaling through the hGITRL-GITR pathway, as opposed to the maximal achievable level.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

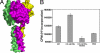
Similar articles
-
Human glucocorticoid-induced TNF receptor ligand regulates its signaling activity through multiple oligomerization states.Proc Natl Acad Sci U S A. 2008 Apr 8;105(14):5465-70. doi: 10.1073/pnas.0711350105. Epub 2008 Mar 31. Proc Natl Acad Sci U S A. 2008. PMID: 18378892 Free PMC article.
-
Evolution of GITRL immune function: murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily.Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):635-40. doi: 10.1073/pnas.0710529105. Epub 2008 Jan 8. Proc Natl Acad Sci U S A. 2008. PMID: 18182486 Free PMC article.
-
Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR.Curr Biol. 1999 Feb 25;9(4):215-8. doi: 10.1016/s0960-9822(99)80093-1. Curr Biol. 1999. PMID: 10074428
-
Role of the glucocorticoid-induced TNFR-related protein (GITR)-GITR ligand pathway in innate and adaptive immunity.Crit Rev Immunol. 2010;30(6):547-57. doi: 10.1615/critrevimmunol.v30.i6.40. Crit Rev Immunol. 2010. PMID: 21175417 Review.
-
Pharmacological modulation of GITRL/GITR system: therapeutic perspectives.Br J Pharmacol. 2012 Apr;165(7):2089-99. doi: 10.1111/j.1476-5381.2011.01753.x. Br J Pharmacol. 2012. PMID: 22029729 Free PMC article. Review.
Cited by
-
Secreted and transmembrane 1A is a novel co-stimulatory ligand.PLoS One. 2013 Sep 10;8(9):e73610. doi: 10.1371/journal.pone.0073610. eCollection 2013. PLoS One. 2013. PMID: 24039998 Free PMC article.
-
Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation.Clin Dev Immunol. 2010;2010:239083. doi: 10.1155/2010/239083. Epub 2010 Sep 26. Clin Dev Immunol. 2010. PMID: 20936139 Free PMC article. Review.
-
An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy.Nat Cancer. 2022 Mar;3(3):337-354. doi: 10.1038/s43018-022-00334-9. Epub 2022 Mar 7. Nat Cancer. 2022. PMID: 35256819 Free PMC article.
-
Application of nSMOL coupled with LC-MS bioanalysis for monitoring the Fc-fusion biopharmaceuticals Etanercept and Abatacept in human serum.Pharmacol Res Perspect. 2018 Jul 24;6(4):e00422. doi: 10.1002/prp2.422. eCollection 2018 Jul. Pharmacol Res Perspect. 2018. PMID: 30062014 Free PMC article.
-
Advancing targeted co-stimulation with antibody-fusion proteins by introducing TNF superfamily members in a single-chain format.Oncoimmunology. 2016 Sep 27;5(11):e1238540. doi: 10.1080/2162402X.2016.1238540. eCollection 2016. Oncoimmunology. 2016. PMID: 27999756 Free PMC article.
References
-
- Shevach EM, Stephens GL. Nat Rev Immunol. 2006;6:613–618. - PubMed
-
- Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. Eur J Immunol. 2007;37:1165–1169. - PubMed
-
- Watts TH. Annu Rev Immunol. 2005;23:23–68. - PubMed
-
- Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. J Immunol. 2004;173:5008–5020. - PubMed
-
- Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. Eur J Immunol. 2004;34:613–622. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
- DK77500/DK/NIDDK NIH HHS/United States
- DK64315/DK/NIDDK NIH HHS/United States
- DK065247/DK/NIDDK NIH HHS/United States
- R56 AI007289/AI/NIAID NIH HHS/United States
- R21 DK077500/DK/NIDDK NIH HHS/United States
- R01 DK064315/DK/NIDDK NIH HHS/United States
- P01 DK052956/DK/NIDDK NIH HHS/United States
- DK20541/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- DK52956/DK/NIDDK NIH HHS/United States
- AI07289/AI/NIAID NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- R01 DK065247/DK/NIDDK NIH HHS/United States
- R01 AI007289/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

