Acetylcholine receptor gating at extracellular transmembrane domain interface: the "pre-M1" linker
- PMID: 18040058
- PMCID: PMC2151659
- DOI: 10.1085/jgp.200709857
Acetylcholine receptor gating at extracellular transmembrane domain interface: the "pre-M1" linker
Abstract
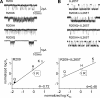
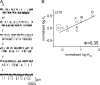
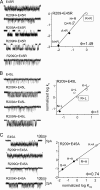
Charged residues in the beta10-M1 linker region ("pre-M1") are important in the expression and function of neuromuscular acetylcholine receptors (AChRs). The perturbation of a salt bridge between pre-M1 residue R209 and loop 2 residue E45 has been proposed as being a principle event in the AChR gating conformational "wave." We examined the effects of mutations to all five residues in pre-M1 (positions M207-P211) plus E45 in loop 2 in the mouse alpha(1)-subunit. M207, Q208, and P211 mutants caused small (approximately threefold) changes in the gating equilibrium constant (K(eq)), but the changes for R209, L210, and E45 were larger. Of 19 different side chain substitutions at R209 on the wild-type background, only Q, K, and H generated functional channels, with the largest change in K(eq) (67-fold) from R209Q. Various R209 mutants were functional on different E45 backgrounds: H, Q, and K (E45A), H, A, N, and Q (E45R), and K, A, and N (E45L). Phi values for R209 (on the E45A background), L210, and E45 were 0.74, 0.35, and 0.80, respectively. Phi values for R209 on the wt and three other backgrounds could not be estimated because of scatter. The average coupling energy between 209/45 side chains (six different pairs) was only -0.33 kcal/mol (for both alpha subunits, combined). Pre-M1 residues are important for expression of functional channels and participate in gating, but the relatively modest changes in closed- vs. open-state energy caused mutations, the weak coupling energy between these residues and the functional activity of several unmatched-charge pairs are not consistent with the perturbation of a salt bridge between R209 and E45 playing the principle role in gating.
Figures


Similar articles
-
Acetylcholine receptor gating at extracellular transmembrane domain interface: the cys-loop and M2-M3 linker.J Gen Physiol. 2007 Dec;130(6):547-58. doi: 10.1085/jgp.200709856. J Gen Physiol. 2007. PMID: 18040057 Free PMC article.
-
Acetylcholine receptor gating: movement in the alpha-subunit extracellular domain.J Gen Physiol. 2007 Dec;130(6):569-79. doi: 10.1085/jgp.200709858. J Gen Physiol. 2007. PMID: 18040059 Free PMC article.
-
Subunit symmetry at the extracellular domain-transmembrane domain interface in acetylcholine receptor channel gating.J Biol Chem. 2010 Dec 10;285(50):38898-904. doi: 10.1074/jbc.M110.169110. Epub 2010 Sep 23. J Biol Chem. 2010. PMID: 20864527 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
The gating isomerization of neuromuscular acetylcholine receptors.J Physiol. 2010 Feb 15;588(Pt 4):573-86. doi: 10.1113/jphysiol.2009.182774. Epub 2009 Nov 23. J Physiol. 2010. PMID: 19933754 Free PMC article. Review.
Cited by
-
Linking the acetylcholine receptor-channel agonist-binding sites with the gate.Biophys J. 2010 Aug 4;99(3):798-807. doi: 10.1016/j.bpj.2010.05.008. Biophys J. 2010. PMID: 20682257 Free PMC article.
-
Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):966-71. doi: 10.1073/pnas.1314997111. Epub 2013 Dec 23. Proc Natl Acad Sci U S A. 2014. PMID: 24367074 Free PMC article.
-
In Silico Modeling of the α7 Nicotinic Acetylcholine Receptor: New Pharmacological Challenges Associated with Multiple Modes of Signaling.Mini Rev Med Chem. 2020;20(10):841-864. doi: 10.2174/1389557520666200130105256. Mini Rev Med Chem. 2020. PMID: 32000651 Free PMC article. Review.
-
Allosteric potentiation of a ligand-gated ion channel is mediated by access to a deep membrane-facing cavity.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10672-10677. doi: 10.1073/pnas.1809650115. Epub 2018 Oct 1. Proc Natl Acad Sci U S A. 2018. PMID: 30275330 Free PMC article.
-
Energetic contributions to channel gating of residues in the muscle nicotinic receptor β1 subunit.PLoS One. 2013 Oct 23;8(10):e78539. doi: 10.1371/journal.pone.0078539. eCollection 2013. PLoS One. 2013. PMID: 24194945 Free PMC article.
References
-
- Brejc, K., W.J. van Dijk, R.V. Klaassen, M. Schuurmans, J. van Der Oost, A.B. Smit, and T.K. Sixma. 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276. - PubMed
-
- Castaldo, P., P. Stefanoni, F. Miceli, G. Coppola, E.M. Del Giudice, G. Bellini, A. Pascotto, J.R. Trudell, N.L. Harrison, L. Annunziato, and M. Taglialatela. 2004. A novel hyperekplexia-causing mutation in the pre-transmembrane segment 1 of the human glycine receptor α1 subunit reduces membrane expression and impairs gating by agonists. J. Biol. Chem. 279:25598–25604. - PubMed

