Acetylcholine receptor gating: movement in the alpha-subunit extracellular domain
- PMID: 18040059
- PMCID: PMC2151656
- DOI: 10.1085/jgp.200709858
Acetylcholine receptor gating: movement in the alpha-subunit extracellular domain
Abstract
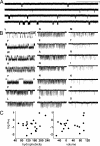
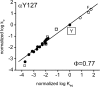
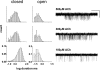
Acetylcholine receptor channel gating is a brownian conformational cascade in which nanometer-sized domains ("Phi blocks") move in staggering sequence to link an affinity change at the transmitter binding sites with a conductance change in the pore. In the alpha-subunit, the first Phi-block to move during channel opening is comprised of residues near the transmitter binding site and the second is comprised of residues near the base of the extracellular domain. We used the rate constants estimated from single-channel currents to infer the gating dynamics of Y127 and K145, in the inner and outer sheet of the beta-core of the alpha-subunit. Y127 is at the boundary between the first and second Phi blocks, at a subunit interface. alphaY127 mutations cause large changes in the gating equilibrium constant and with a characteristic Phi-value (Phi = 0.77) that places this residue in the second Phi-block. We also examined the effect on gating of mutations in neighboring residues deltaI43 (Phi = 0.86), epsilonN39 (complex kinetics), alphaI49 (no effect) and in residues that are homologous to alphaY127 on the epsilon, beta, and delta subunits (no effect). The extent to which alphaY127 gating motions are coupled to its neighbors was estimated by measuring the kinetic and equilibrium constants of constructs having mutations in alphaY127 (in both alpha subunits) plus residues alphaD97 or deltaI43. The magnitude of the coupling between alphaD97 and alphaY127 depended on the alphaY127 side chain and was small for both H (0.53 kcal/mol) and C (-0.37 kcal/mol) substitutions. The coupling across the single alpha-delta subunit boundary was larger (0.84 kcal/mol). The Phi-value for K145 (0.96) indicates that its gating motion is correlated temporally with the motions of residues in the first Phi-block and is not synchronous with those of alphaY127. This suggests that the inner and outer sheets of the alpha-subunit beta-core do not rotate as a rigid body.
Figures
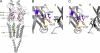


Similar articles
-
Acetylcholine receptor gating at extracellular transmembrane domain interface: the "pre-M1" linker.J Gen Physiol. 2007 Dec;130(6):559-68. doi: 10.1085/jgp.200709857. J Gen Physiol. 2007. PMID: 18040058 Free PMC article.
-
Acetylcholine receptor gating at extracellular transmembrane domain interface: the cys-loop and M2-M3 linker.J Gen Physiol. 2007 Dec;130(6):547-58. doi: 10.1085/jgp.200709856. J Gen Physiol. 2007. PMID: 18040057 Free PMC article.
-
Linking the acetylcholine receptor-channel agonist-binding sites with the gate.Biophys J. 2010 Aug 4;99(3):798-807. doi: 10.1016/j.bpj.2010.05.008. Biophys J. 2010. PMID: 20682257 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
The gating isomerization of neuromuscular acetylcholine receptors.J Physiol. 2010 Feb 15;588(Pt 4):573-86. doi: 10.1113/jphysiol.2009.182774. Epub 2009 Nov 23. J Physiol. 2010. PMID: 19933754 Free PMC article. Review.
Cited by
-
Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity.J Biol Chem. 2011 Sep 2;286(35):31043-31054. doi: 10.1074/jbc.M111.262014. Epub 2011 Jul 14. J Biol Chem. 2011. PMID: 21757735 Free PMC article.
-
Glycine hinges with opposing actions at the acetylcholine receptor-channel transmitter binding site.Mol Pharmacol. 2011 Mar;79(3):351-9. doi: 10.1124/mol.110.068767. Epub 2010 Nov 29. Mol Pharmacol. 2011. PMID: 21115636 Free PMC article.
-
Acetylcholine receptor gating at extracellular transmembrane domain interface: the "pre-M1" linker.J Gen Physiol. 2007 Dec;130(6):559-68. doi: 10.1085/jgp.200709857. J Gen Physiol. 2007. PMID: 18040058 Free PMC article.
-
Energetics of gating at the apo-acetylcholine receptor transmitter binding site.J Gen Physiol. 2010 Apr;135(4):321-31. doi: 10.1085/jgp.200910384. J Gen Physiol. 2010. PMID: 20351060 Free PMC article.
-
Gating mechanisms in Cys-loop receptors.Eur Biophys J. 2009 Dec;39(1):37-49. doi: 10.1007/s00249-009-0452-y. Epub 2009 Apr 29. Eur Biophys J. 2009. PMID: 19404635 Review.

