Immunolocalization of Periostin-like factor and Periostin during embryogenesis
- PMID: 18040074
- PMCID: PMC2326108
- DOI: 10.1369/jhc.7A7321.2007
Immunolocalization of Periostin-like factor and Periostin during embryogenesis
Abstract
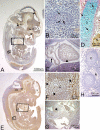
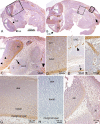
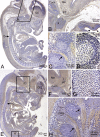
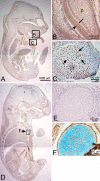
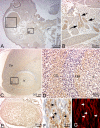
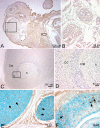
Periostin-like factor (PLF) and Periostin are alternatively spliced mRNAs. Our findings are the first to show similarities and differences between PLF and Periostin location using isoform-specific antibodies. The differences in when and where they are present during mouse embryogenesis suggest that they may have different functions. Using immunostaining techniques, we observed that PLF was highly expressed at 12.5 days postconception (dpc) in the intermediate and outer zones of most brain regions, spinal cord, cranial and spinal nerves, and chondrocytes in developing bone and in the heart wall. By 16.5 dpc, PLF was also present in ameloblasts and odontoblasts in developing teeth, and by 19.5 dpc, PLF was present at low levels only in vagal nerve bundles, discrete white matter bundles in the brain, and chondrocytes of developing ribs. Periostin, on the other hand, was absent at 12.5 dpc from dorsal spinal cord and from cranial and spinal nerves. By 16.5 dpc, Periostin was present in many spinal nerves, but absent thereafter, and at 19.5 dpc, Periostin was present in chondrocytes in developing bone but not in neural tissues. The different spatial and temporal location of PLF and Periostin in cartilage and bone cells suggests different roles for these proteins in endochondral bone formation. The early expression of PLF in brain differentiation zones and in developing axon bundles and nerves suggests that it may facilitate axon growth.
Figures


Similar articles
-
Expression and function of periostin-isoforms in bone.J Cell Biochem. 2004 Aug 1;92(5):1044-61. doi: 10.1002/jcb.20115. J Cell Biochem. 2004. PMID: 15258926
-
Periostin-like-factor in osteogenesis.J Cell Physiol. 2009 Mar;218(3):584-92. doi: 10.1002/jcp.21633. J Cell Physiol. 2009. PMID: 19006175
-
Periostin-like-factor and Periostin in an animal model of work-related musculoskeletal disorder.Bone. 2009 Mar;44(3):502-12. doi: 10.1016/j.bone.2008.11.012. Epub 2008 Nov 27. Bone. 2009. PMID: 19095091 Free PMC article.
-
Introductory review: periostin-gene and protein structure.Cell Mol Life Sci. 2017 Dec;74(23):4259-4268. doi: 10.1007/s00018-017-2643-5. Epub 2017 Sep 7. Cell Mol Life Sci. 2017. PMID: 28884327 Free PMC article. Review.
-
Periostin: a novel prognostic and therapeutic target for genitourinary cancer?Clin Genitourin Cancer. 2014 Oct;12(5):301-11. doi: 10.1016/j.clgc.2014.02.005. Epub 2014 Feb 21. Clin Genitourin Cancer. 2014. PMID: 24656869 Review.
Cited by
-
Periostin Promotes Neural Stem Cell Proliferation and Differentiation following Hypoxic-Ischemic Injury.PLoS One. 2015 Apr 20;10(4):e0123585. doi: 10.1371/journal.pone.0123585. eCollection 2015. PLoS One. 2015. PMID: 25894199 Free PMC article.
-
The role of periostin in tissue remodeling across health and disease.Cell Mol Life Sci. 2014 Apr;71(7):1279-88. doi: 10.1007/s00018-013-1494-y. Epub 2013 Oct 22. Cell Mol Life Sci. 2014. PMID: 24146092 Free PMC article. Review.
-
Astroglial-derived periostin promotes axonal regeneration after spinal cord injury.J Neurosci. 2014 Feb 12;34(7):2438-43. doi: 10.1523/JNEUROSCI.2947-13.2014. J Neurosci. 2014. PMID: 24523534 Free PMC article.
-
Functions of Periostin in dental tissues and its role in periodontal tissues' regeneration.Cell Mol Life Sci. 2017 Dec;74(23):4279-4286. doi: 10.1007/s00018-017-2645-3. Epub 2017 Sep 9. Cell Mol Life Sci. 2017. PMID: 28889194 Free PMC article. Review.
-
Serum periostin levels in early in pregnancy are significantly altered in women with miscarriage.Reprod Biol Endocrinol. 2017 Nov 2;15(1):87. doi: 10.1186/s12958-017-0307-9. Reprod Biol Endocrinol. 2017. PMID: 29096644 Free PMC article.
References
-
- Billings PC, Herrick DJ, Kucich U, Engelsberg BN, Abrams WR, Macarak EJ, Rosenbloom J, et al. (2000) Extracellular matrix and nuclear localization of beta ig-h3 in human bladder smooth muscle and fibroblast cells. J Cell Biochem 79:261–273 - PubMed
-
- el Ghouzzi V, Le Merrer M, Perrin-Schmitt F, Lajeunie E, Benit P, Renier D, Bourgeois P, et al. (1997) Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet 15:42–46 - PubMed
-
- Ferguson JW, Mikesh MF, Wheeler EF, LeBaron RG (2003) Developmental expression patterns of Beta-ig (betaIG-H3) and its function as a cell adhesion protein. Mech Dev 120:851–864 - PubMed
-
- Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ (2003) Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics 14:261–271 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

