Immunolocalization of a novel collectin CL-K1 in murine tissues
- PMID: 18040075
- PMCID: PMC2324184
- DOI: 10.1369/jhc.7A7312.2007
Immunolocalization of a novel collectin CL-K1 in murine tissues
Abstract
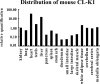
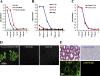
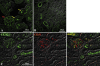
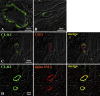
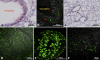
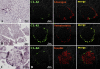
We have recently identified a novel collectin, CL-K1, that may play a role in innate immunity as a member of the collectin family. In this study using mice, we investigated the tissue distribution of CL-K1 for better understanding of its pathophysiological relevance. Real-time PCR analyses demonstrated that CL-K1 mRNA was expressed in all tissues tested. Immunohistochemical analyses demonstrated that CL-K1 was expressed in proximal tubules of kidney, in mucosa of the gastrointestinal tract, and in bronchial glands of bronchioles similar to the localization of SP-A and SP-D in these pulmonary structures. Immunohistochemistry also showed that CL-K1 was highly expressed in hepatocytes around the central veins in liver, which suggests that murine CL-K1 may be mainly produced in the liver and secreted into the blood stream as is human CL-K1. CL-K1 was especially detected in vascular smooth muscle in several types of tissues. In addition, it was also expressed in intestinal Paneth cells, in mesangial cells of kidney, in pancreatic islet D cells, and in neurons of the brain. It is of interest that this profile of CL-K1 expression is unique among the collectins. Together these histological findings may be useful for understanding the biological function of this novel collectin.
Figures

Similar articles
-
Comparison of human blood concentrations of collectin kidney 1 and mannan-binding lectin.J Biochem. 2012 Jan;151(1):57-64. doi: 10.1093/jb/mvr114. Epub 2011 Sep 4. J Biochem. 2012. PMID: 21893516
-
Identification and characterization of a novel human collectin CL-K1.Microbiol Immunol. 2006;50(12):1001-13. doi: 10.1111/j.1348-0421.2006.tb03868.x. Microbiol Immunol. 2006. PMID: 17179669
-
CL-L1 and CL-K1 Exhibit Widespread Tissue Distribution With High and Co-Localized Expression in Secretory Epithelia and Mucosa.Front Immunol. 2018 Jul 31;9:1757. doi: 10.3389/fimmu.2018.01757. eCollection 2018. Front Immunol. 2018. PMID: 30108587 Free PMC article.
-
Biological functions of the novel collectins CL-L1, CL-K1, and CL-P1.J Biomed Biotechnol. 2012;2012:493945. doi: 10.1155/2012/493945. Epub 2012 Apr 11. J Biomed Biotechnol. 2012. PMID: 22570530 Free PMC article. Review.
-
The collectins CL-L1, CL-K1 and CL-P1, and their roles in complement and innate immunity.Immunobiology. 2016 Oct;221(10):1058-67. doi: 10.1016/j.imbio.2016.05.012. Epub 2016 Jun 2. Immunobiology. 2016. PMID: 27377710 Review.
Cited by
-
Human stem cell-derived retinal epithelial cells activate complement via collectin 11 in response to stress.Sci Rep. 2017 Nov 7;7(1):14625. doi: 10.1038/s41598-017-15212-z. Sci Rep. 2017. PMID: 29116192 Free PMC article.
-
Collectin Kidney 1 Plays an Important Role in Innate Immunity against Streptococcus pneumoniae Infection.J Innate Immun. 2017;9(2):217-228. doi: 10.1159/000453316. Epub 2017 Jan 10. J Innate Immun. 2017. PMID: 28068663 Free PMC article.
-
Collectin-11 Is an Important Modulator of Retinal Pigment Epithelial Cell Phagocytosis and Cytokine Production.J Innate Immun. 2017;9(6):529-545. doi: 10.1159/000478042. Epub 2017 Aug 4. J Innate Immun. 2017. PMID: 28772263 Free PMC article.
-
Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury.J Clin Invest. 2016 May 2;126(5):1911-25. doi: 10.1172/JCI83000. Epub 2016 Apr 18. J Clin Invest. 2016. PMID: 27088797 Free PMC article.
-
Molecular basis of sugar recognition by collectin-K1 and the effects of mutations associated with 3MC syndrome.BMC Biol. 2015 Apr 17;13:27. doi: 10.1186/s12915-015-0136-2. BMC Biol. 2015. PMID: 25912189 Free PMC article.
References
-
- Andersen O, Friis P, Holm Nielsen E, Vilsgaard K, Leslie RG, Svehag SE (1992) Purification, subunit characterization and ultrastructure of three soluble bovine lectins: conglutinin, mannose-binding protein and the pentraxin serum amyloid P-component. Scand J Immunol 36:131–141 - PubMed
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ (2000) Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1:99–100 - PubMed
-
- Drickamer K (1988) Two distinct classes of carbohydrate recognition domains in animal lectins. J Biol Chem 263:9557–9560 - PubMed
-
- Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ (1987) The major lung surfactant protein, SP28–36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem 262:13877–13880 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

