Running promotes wakefulness and increases cataplexy in orexin knockout mice
- PMID: 18041476
- PMCID: PMC2082091
- DOI: 10.1093/sleep/30.11.1417
Running promotes wakefulness and increases cataplexy in orexin knockout mice
Abstract
Study objective: People with narcolepsy and mice lacking orexin/hypocretin have disrupted sleep/wake behavior and reduced physical activity. Our objective was to identify physiologic mechanisms through which orexin deficiency reduces locomotor activity.
Design: We examined spontaneous wheel running activity and its relationship to sleep/wake behavior in wild type (WT) and orexin knockout (KO) mice. Additionally, given that physical activity promotes alertness, we also studied whether orexin deficiency reduces the wake-promoting effects of exercise.
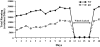
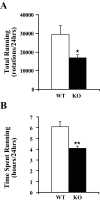
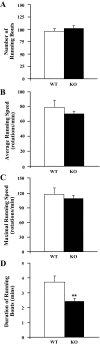
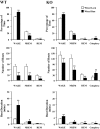
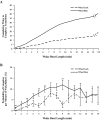
Measurements and results: Orexin KO mice ran 42% less than WT mice. Their ability to run appeared normal as they initiated running as often as WT mice and ran at normal speeds. However, their running bouts were considerably shorter, and they often had cataplexy or quick transitions into sleep after running. Wheel running increased the total amount of wakefulness in WT and orexin KO mice similarly, however, KO mice continued to have moderately fragmented sleep/wake behavior. Wheel running also doubled the amount of cataplexy by increasing the probability of transitioning into cataplexy.
Conclusions: Orexin KO mice run significantly less than normal, likely due to sleepiness, imminent cataplexy, or a reduced motivation to run. Orexin is not required for the wake-promoting effects of wheel running given that both WT and KO mice had similar increases in wakefulness with running wheels. In addition, the clear increase in cataplexy with wheel running suggests the possibility that positive emotions or reward can trigger murine cataplexy, similar to that seen in people and dogs with narcolepsy.
Figures



References
-
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Med. 2000;6:991–7. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

