Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude
- PMID: 18041485
- PMCID: PMC2082097
- DOI: 10.1093/sleep/30.11.1527
Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude
Abstract
Study objectives: To assess the effects of zolpidem and zaleplon on nocturnal sleep and breathing patterns at altitude, as well as on daytime attention, fatigue, and sleepiness.
Design: Double-blind, randomized, placebo-controlled, cross-over trial.
Setting: 3 day and night alpine expedition at 3,613 m altitude.
Participants: 12 healthy male trekkers.
Procedure: One week spent at 1,000 m altitude (baseline control), followed by 3 periods of 3 consecutive treatment nights (N1-3) at altitude, to test 10 mg zolpidem, 10 mg zaleplon, and placebo given at 21:45.
Measures: Sleep from EEG, actigraphy and sleep logs; overnight arterial saturation in oxygen (SpO2) from infrared oximetry; daytime attention, fatigue and sleepiness from a Digit Symbol Substitution Test, questionnaires, and sleep logs; acute mountain sickness (AMS) from the Lake Louise questionnaire.
Results: Compared to baseline control, sleep at altitude was significantly impaired in placebo subjects as shown by an increase in the amount of Wakefulness After Sleep Onset (WASO) from 17 +/- 8 to 36 +/- 13 min (P<0.05) and in arousals from 5 +/- 3 to 20 +/- 8 (P<0.01). Slow wave sleep (SWS) and stage 4 respectively decreased from 26.7% +/- 5.8% to 20.6% +/- 5.8% of total sleep time (TST) and from 18.2% +/- 5.2% to 12.4% +/- 3.1% TST (P<0.05 and P<0.001, respectively). Subjects also complained from a feeling of poor sleep quality combined with numerous 02 desaturation episodes. Subjective fatigue and AMS score were increased. Compared to placebo control, WASO decreased by approximately 6 min (P<0.05) and the sleep efficiency index increased by 2% (P<0.01) under zaleplon and zolpidem, while SWS and stage 4 respectively increased to 22.5% +/- 5.4% TST (P<0.05) and to 15.0% +/- 3.4% TST (P<0.0001) with zolpidem only; both drugs further improved sleep quality. No adverse effect on nighttime SpO2, daytime attention level, alertness, or mood was observed under either hypnotic. AMS was also found to be reduced under both medications.
Conclusions: Both zolpidem and zaleplon have positive effects on sleep at altitude without adversely affecting respiration, attention, alertness, or mood. Hence, they may be safely used by climbers.
Figures
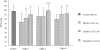

References
-
- Anholm JD, Powles AC, Downey R, 3rd, et al. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis. 1992;145:817–26. - PubMed
-
- Nicholson AN, Smith PA, Stone BM, Bradwell AR, Coote JH. Altitude insomnia: studies during an expedition to the Himalayas. Sleep. 1988;11:354–61. - PubMed
-
- Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. Respiration and abnormal sleep in patients with congestive heart failure. Chest. 1989;96:480–8. - PubMed
-
- Khoo MCK, Gottschalk A, Pack A. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol. 1991;70:2014–24. - PubMed
-
- Downey R, Bonnet MH. Performance during frequent sleep disruption. Sleep. 1987;10:354–63. - PubMed

