Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia
- PMID: 18041488
- PMCID: PMC2082089
- DOI: 10.1093/sleep/30.11.1555
Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia
Abstract
Study objectives: To evaluate the efficacy and safety of doxepin 1, 3, and 6 mg in insomnia patients.
Design: Adults (18-64 y) with chronic primary insomnia (DSM-IV) were randomly assigned to one of four sequences of 1 mg, 3 mg, and 6 mg of doxepin, and placebo in a crossover study. Treatment periods consisted of 2 polysomnographic assessment nights with a 5-day or 12-day drug-free interval between periods. Efficacy was assessed using polysomnography (PSG) and patient-reported measures. Safety analyses included measures of residual sedation and adverse events.
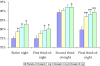
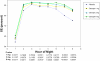
Measurements and results: Sixty-seven patients were randomized. Wake time during sleep, the a priori defined primary endpoint, was statistically significantly improved at the doxepin 3 mg and 6 mg doses versus placebo. All three doses had statistically significant improvements versus placebo for PSG-defined wake after sleep onset, total sleep time, and overall sleep efficiency (SE). SE in the final third-of-the-night also demonstrated statistically significant improvement at all doses. The doxepin 6 mg dose significantly reduced subjective latency to sleep onset. All three doxepin doses had a safety profile comparable to placebo. There were no statistically significant differences in next-day residual sedation, and sleep architecture was generally clinically preserved.
Conclusions: In adults with primary insomnia, doxepin 1 mg, 3 mg, and 6 mg was well-tolerated and produced improvement in objective and subjective sleep maintenance and duration endpoints that persisted into the final hour of the night. The side-effect profile was comparable to placebo, with no reported anticholinergic effects, no memory impairment, and no significant hangover/next-day residual effects. These data demonstrate that doxepin 1 mg, 3 mg, and 6 mg is efficacious in improving the sleep of patients with chronic primary insomnia.
Figures
References
-
- Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 2004;39:411–8. - PubMed
-
- Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31:333–46. - PubMed
-
- Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: age, gender, and ethnicity. Mahwah, NJ: Erlbaum; 2004.
-
- Buysse D, Reynolds CF, III, Kupfer DJ, et al. Effects of diagnosis on treatment recommendations in chronic insomnia-a report from the APA/NIMH DSM-IV field trial. Sleep. 1997;20:542–52. - PubMed
-
- Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical