Dystrobrevin and dystrophin family gene expression in zebrafish
- PMID: 18042440
- PMCID: PMC3360968
- DOI: 10.1016/j.modgep.2007.10.004
Dystrobrevin and dystrophin family gene expression in zebrafish
Abstract
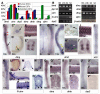
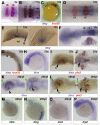
Dystrophin/dystrobrevin superfamily proteins play structural and signalling roles at the plasma membrane of many cell types. Defects in them or the associated multiprotein complex cause a range of neuromuscular disorders. Members of the dystrophin branch of the family form heterodimers with members of the dystrobrevin branch, mediated by their coiled-coil domains. To determine which combinations of these proteins might interact during embryonic development, we set out to characterise the gene expression pattern of dystrophin and dystrobrevin family members in zebrafish. gamma-dystrobrevin (dtng), a novel dystrobrevin recently identified in fish, is the predominant form of dystrobrevin in embryonic development. Dtng and dmd (dystrophin) have similar spatial and temporal expression patterns in muscle, where transcripts are localized to the ends of differentiated fibres at the somite borders. Dtng is expressed in the notochord while dmd is expressed in the chordo-neural hinge and then in floor plate and hypochord. In addition, dtng is dynamically expressed in rhombomeres 2 and 4-6 of the hindbrain and in the ventral midbrain. alpha-dystrobrevin (dtna) is expressed widely in the brain with particularly strong expression in the hypothalamus and the telencephalon; drp2 is also expressed widely in the brain. Utrophin expression is found in early pronephros and lateral line development and utrophin and dystrophin are both expressed later in the gut. beta-dystrobrevin (dtnb) is expressed in the pronephric duct and widely at low levels. In summary, we find clear instances of co-expression of dystrophin and dystrobrevin family members in muscle, brain and pronephric duct development and many examples of strong and specific expression of members of one family but not the other, an intriguing finding given the presumed heterodimeric state of these molecules.
Figures

References
-
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. - PubMed
-
- Bassett DI, Bryson-Richardson RJ, Daggett DF, Gautier P, Keenan DG, Currie PD. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development. 2003;130:5851–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

